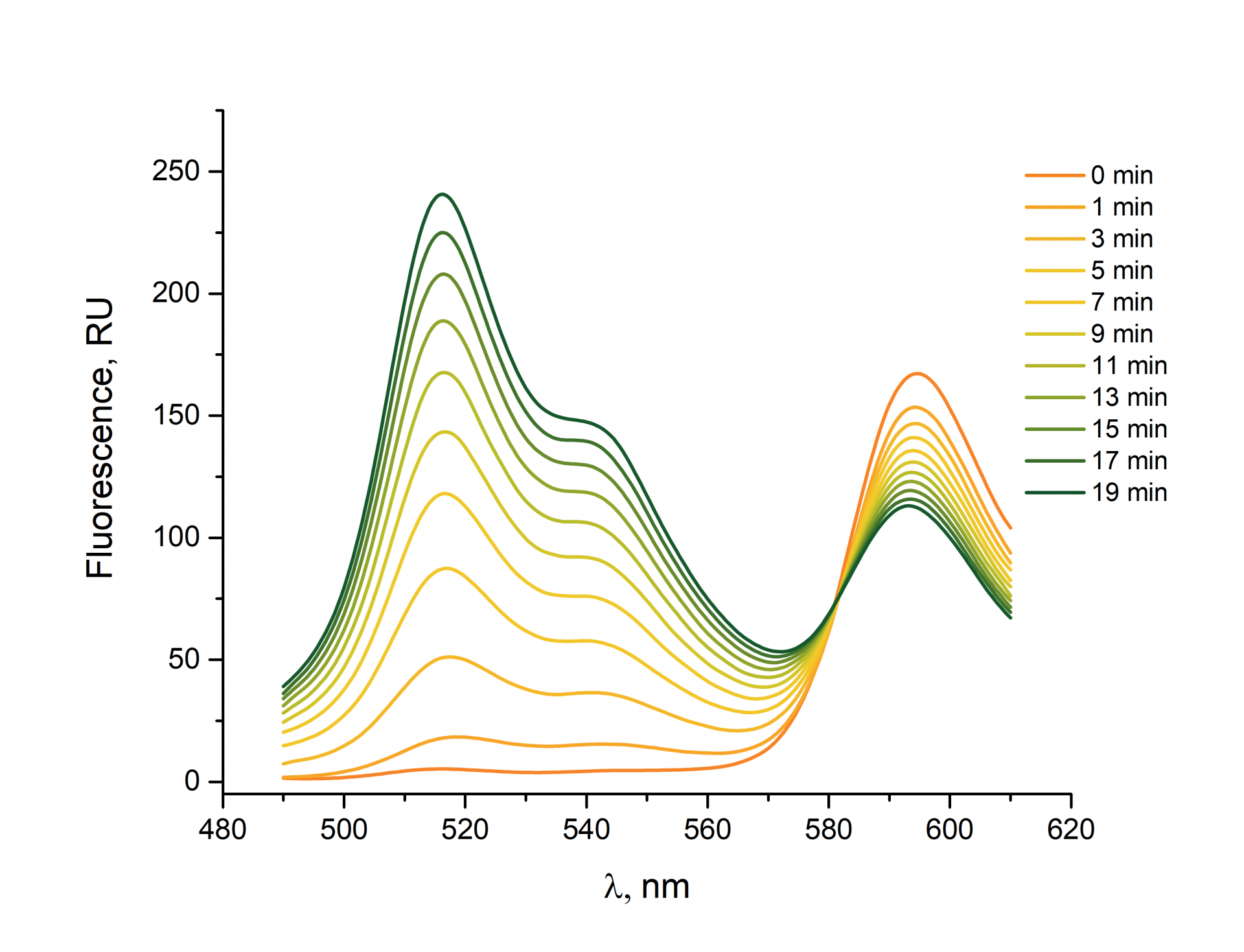
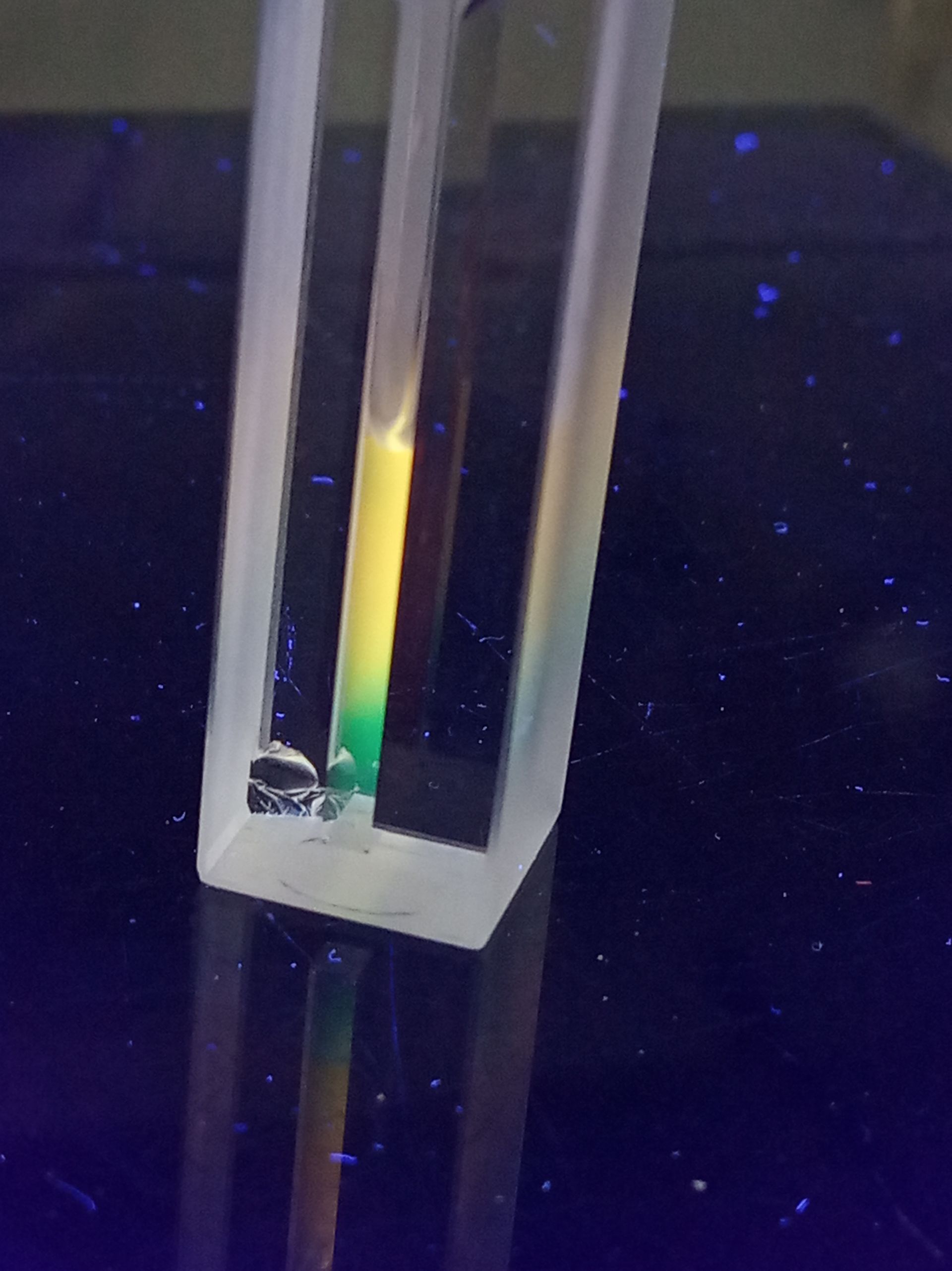
BDP 581/591 is a fluorophore that can be used for ROS detection. In a reduced state, the fluorophore exhibits a fluorescence emission maximum at 591 nm. Once the fluorophore has been oxidized by reactive oxygen species, the fluorescence emission maximum shifts to 515 nm.
General recommendations for experiments performed on living cells by flow cytometry using BDP 581/591 dye:
The following recommendations were developed on the basis of the results of the experiments on NS0 cells. We recommend determining the optimal concentrations of the dye, ROS inducer, antioxidant, and experimental conditions for your cell type and current task.
1. Both suspension and adherent cell cultures can be used. Cells should be grown in complete media but incubated with the dye, a ROS inducer, and an antioxidant in PBS.
2. We strongly recommend including positive and negative controls
✔ TBHP* (tert-butyl hydroperoxide, inducer of ROS) can be used as a positive control.
✔ Cells pre-treated with an antioxidant (α-tocopherol or butylated hydroxytoluene**) followed treating with TBHP can be used as a negative control.
3. Recommended final concentration of BDP 581/591 dye for ROS detection is 5 µM or higher.
4. Incubating for 30 minutes is sufficient for staining with BDP581/591 dye.
5. Fluorescence is to be measured in the FL1 channel (using a 530/30 BP filter)
* Alternatively, Hydrogen peroxide (H2O2) can be used.
** NAC (N-acetyl-L-cysteine) is not recommended as an antioxidant since it showed no antioxidant effect in a few series of experiments with BDP581/591 dye.
An application example for ROS detection using BDP 581/591 dye
The experiment was carried out on NS0 cells. NS0 suspension culture is a model cell line derived from murine myeloma used in biomedical research. Cells were cultivated in RPMI 1640 medium containing 10% Fetal Bovine Serum.
✔ ROS inducer: TBHP (tert-Butyl hydroperoxide)
✔ Antioxidants: α-tocopherol, butylated hydroxytoluene
✔ Detection: BDP 581/591 carboxylic acid, CellROX® Orange Reagent (for comparison purposes)
A solution of α-tocopherol (15 mM) was prepared by dissolving α-tocopherol (3.2 mg) in ethanol 96% (0.5 mL).
A solution of butylated hydroxytoluene (4 mM) was prepared by dissolving butylated hydroxytoluene (900 µg) in ethanol 96% (1 mL).
A solution of BDP 581/591 carboxylic acid (100 mM) was prepared by dissolving BDP 581/591 carboxylic acid (40 mg) in DMSO (1 mL). From this stock solution, a sample with a volume of 2.5 µL was taken and diluted in deionized sterile water (1 mL) to prepare a solution with a concentration of 250 µM.
1. Cells were collected by centrifugation (800 g for 8 min), resuspended in pre-warmed PBS (0.5 mL) and divided into samples (300 thousand cells per sample).
2. The following samples were prepared:
• Antioxidant #1: α-tocopherol (5 µL, 15 mM) was added to the sample (cell suspension, 300 thousand cells) (the final concentration 150 µM).
• Antioxidant #2: butylated hydroxytoluene (1 µL, 4 mM) was added to the sample (cell suspension, 300 thousand cells) (the final concentration 8 µM).
Cells were incubated with the antioxidant for 1 hour at +37ºС. Next, TBHP (2 µL, 50 mM) was added (the final concentration 200 µM), and the samples were incubated for 30 minutes at +37ºС.
• ROS inducer: TBHP (2 µL, 50 mM) was added (the final concentration 200 µM), and the samples were incubated for 30 minutes at +37ºС.
3. Samples were treated with fluorophore:
• BDP 581/591 carboxylic acid (10 µL, 250 µM) was added to the sample (the final concentration 5 µM).
• CellROX® Orange Reagent (1 µL, 250 µM) was added to the sample (fresh solution, the final concentration 500 nM).
4. All samples were incubated for 30 minutes at +37ºС.
5. Flow cytometry was performed using a BD FACSCalibur (BD Biosciences) analyzer. A logarithmic amplification scale using the following configuration of band-pass (BP) filters was applied: 488 nm excitation line, FL1 (530/30 BP for collection of BDP 581/591 fluorescence signals), and FL2 (585/42 BP for collection of CellROX® Orange fluorescence signals). A typical run used a sample with ~10,000 cells.
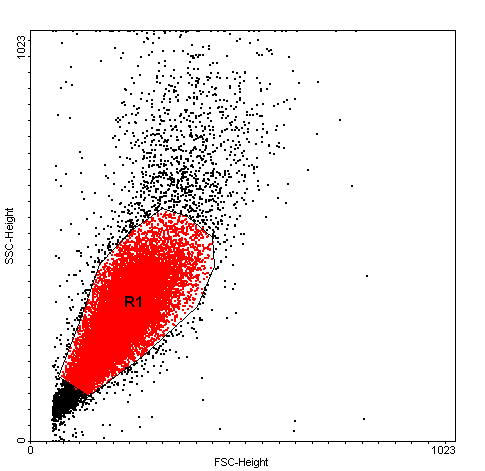
Cells were gated on a forward (FS), and side scatters (SS) dot plot and subsequently analyzed for the median fluorescence intensity of the BDP 581/591 reagent and CellROX® Orange reagent.
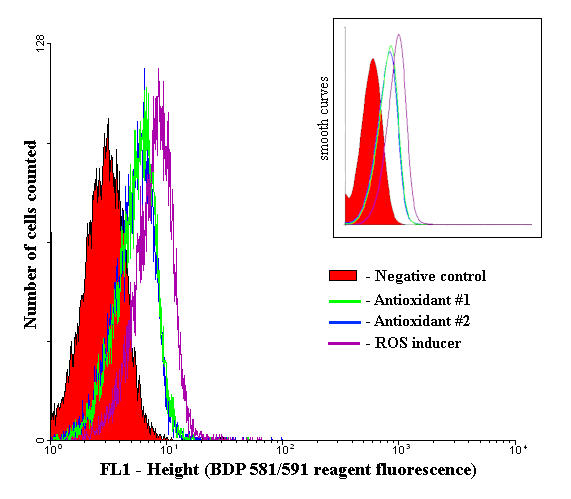
Histogram of fluorescent intensity for BDP 581/591 (final concentration 5 µM).
Cells treated with the TBHP demonstrate a higher fluorescence intensity compared to cells pretreated with antioxidants and control cells.
Negative control - cells treated only with BDP 581/591 reagent (5 µM), but not with any antioxidants or ROS inducers
Antioxidant #1 - cells pretreated with α-tocopherol (150 µM), then treated with TBHP (200 µM), and finally with BDP 581/591 reagent (5 µM)
Antioxidant #2 - cells pretreated with butylated hydroxytoluene (8 µM), then treated with TBHP (200 µM) and finally with BDP 581/591 reagent (5 µM)
ROS inducer - cells treated with TBHP (200 µM) and then with BDP 581/591 reagent (5 µM), but not with any antioxidants
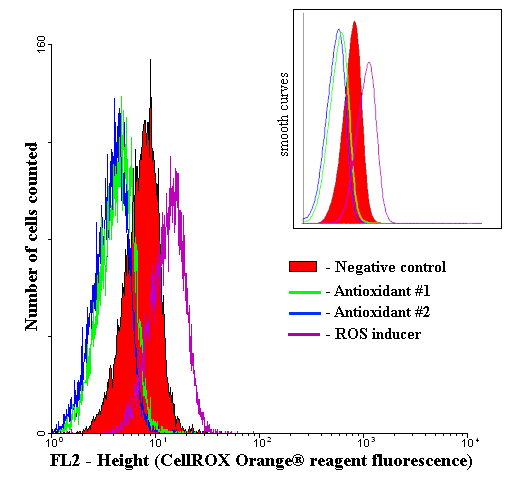
Histogram of fluorescent intensity for CellROX® Orange (final concentration 500 nM)
Cells treated with the TBHP demonstrate a higher fluorescence intensity compared to cells pretreated with antioxidants and control cells.
Negative control - cells treated only with CellROX® Orange reagent (500 nM), but not with any antioxidants or ROS inducers
Antioxidant #1 - cells pretreated with α-tocopherol (150 µM), then treated with TBHP (200 µM), and finally with CellROX® Orange reagent (500 nM)
Antioxidant #2 - cells pretreated with butylated hydroxytoluene (8 µM), then treated with TBHP (200 µM) and finally with CellROX® Orange reagent (500 nM)
ROS inducer - cells treated with TBHP (200 µM) and then with CellROX® Orange reagent (500 nM), but not with any antioxidants
Comparison of the median fluorescence intensity of BDP 581/591 and CellROX® Orange positive cells.
| Reagent | Negative control | Antioxidant #1 α-tocopherol (150 µM) + TBHP (200 µM) | Antioxidant #2 butylated hydroxytoluene (8 µM) + TBHP (200 µM) | ROS inducer TBHP (200 µM) |
| BDP 581/591 | 3.00 | 5.52 | 5.78 | 8.13 |
| CellROX® Orange | 7.64 | 3.89 | 4.41 | 13.70 |
CellROX® is a trademark of Thermo Fisher Scientific