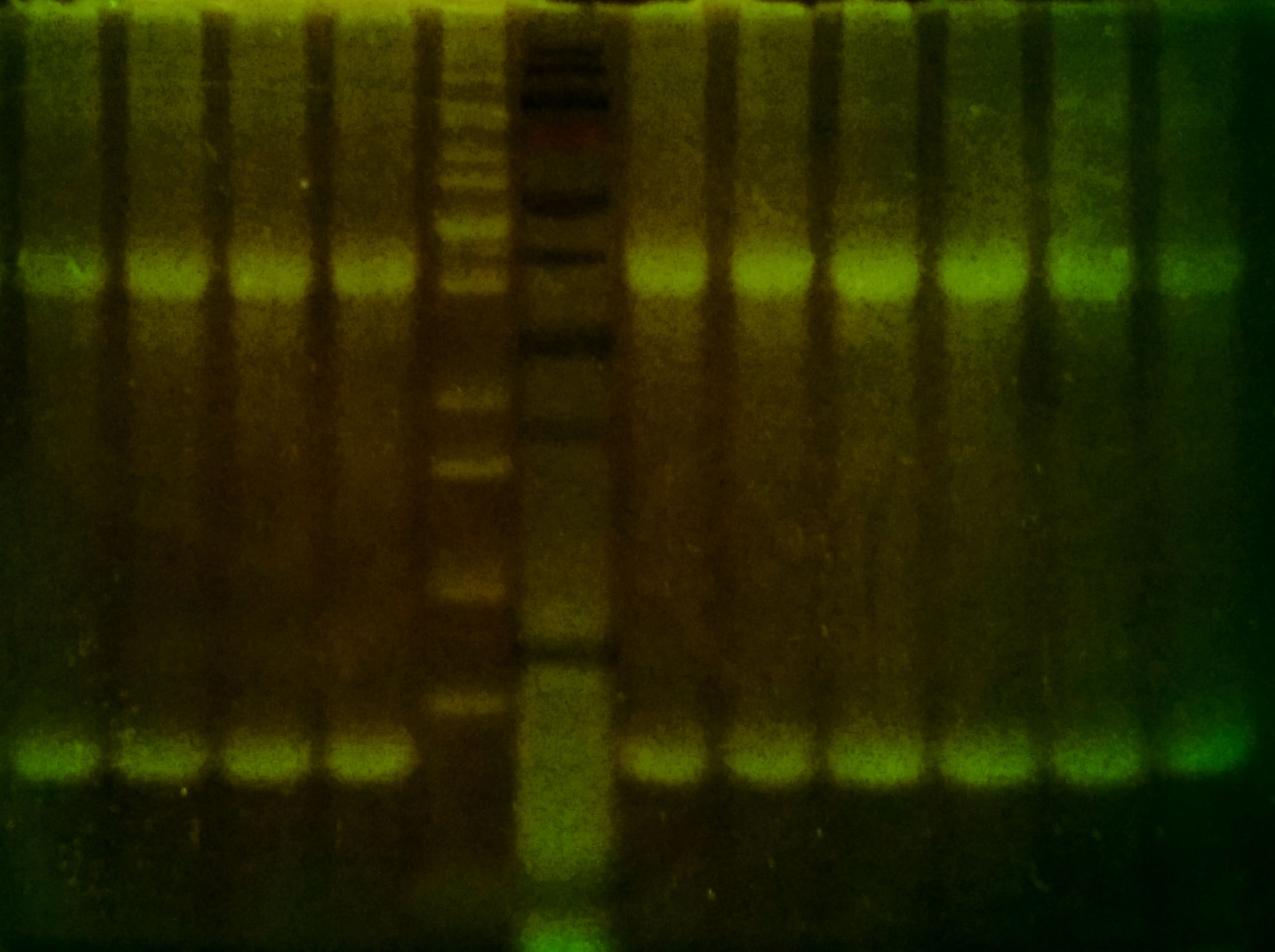
ProteOrange Protein Gel Stain is a fluorescent stain for the visualization of proteins in polyacrylamide gels after electrophoresis, an analog of SYPRO™ Orange. Proteins bands stained with ProteOrange can be excited by ultraviolet light at approximately 300 nm or by visible light at approximately 470 nm and detected at 570 nm using an appropriate filter.
ProteOrange Protein Gel Stain has many advantages:
✔ 10 times more sensitive than Coomassie™ brilliant blue (CBB) staining and relatively similar to traditional silver staining (detection limit is 3 ng of protein per band for ProteOrange, 30 ng for CBB, 0.3 ng for silver staining)
✔ A simple and quick staining procedure (less than 1 hour)
✔ Low protein-to-protein variability: the stain interacts with the SDS coat around proteins in the gel, so it gives more consistent staining between different types of proteins compared to CBB staining
✔ Easy visualization with routinely used UV or blue-light transilluminator or laser scanner
✔ Broad linear range of detection: fluorescence intensity is linear with protein concentration over three orders of magnitude
✔ Selective for proteins: ProteOrange detects proteins down to 6.5 kDa and does not stain nucleic acids or lipopolysaccharides. It also stains glycosylated proteins.
In this article, we demonstrate step-by-step instructions on staining proteins with ProteOrange in polyacrylamide gels after electrophoresis. Detailed protocol for staining proteins with ProteOrange you can also find on our website.
After the electrophoresis has been finished, follow the steps below:
SYPRO™ is a trademark of Thermo Fisher Scientific
Coomassie™ is a trademark of Imperial Chemical Industries