BDP 493/503 is an analog of BODIPY493/503 widely used to specifically label and track lipid droplets. Compared with Nile Red, BDP 493/503 has a narrow emission spectrum, which makes it an ideal fluorophore for multi-labeling experiments.
BDP 505/515, BDP 630/650, and BDP 650/665 have similar properties, so the recommendations for staining with BDP 493/503 given in this article are applicable to other BDP lipid stains.
Possible applications include fluorescent microscopy, flow cytometry, and experiments involving fluorescence microplate readers. Stains can be used on both fixed and living cells.
Key points when staining with BDP 493/503:
✓ Working concentration of BDP 493/503: 1-8 µM (microscopy applications)
✓ Staining for 20 minutes at 37 °C
✓ Excitation by the 488 nm-laser, emission peak at 503 nm
Staining of lipid droplets
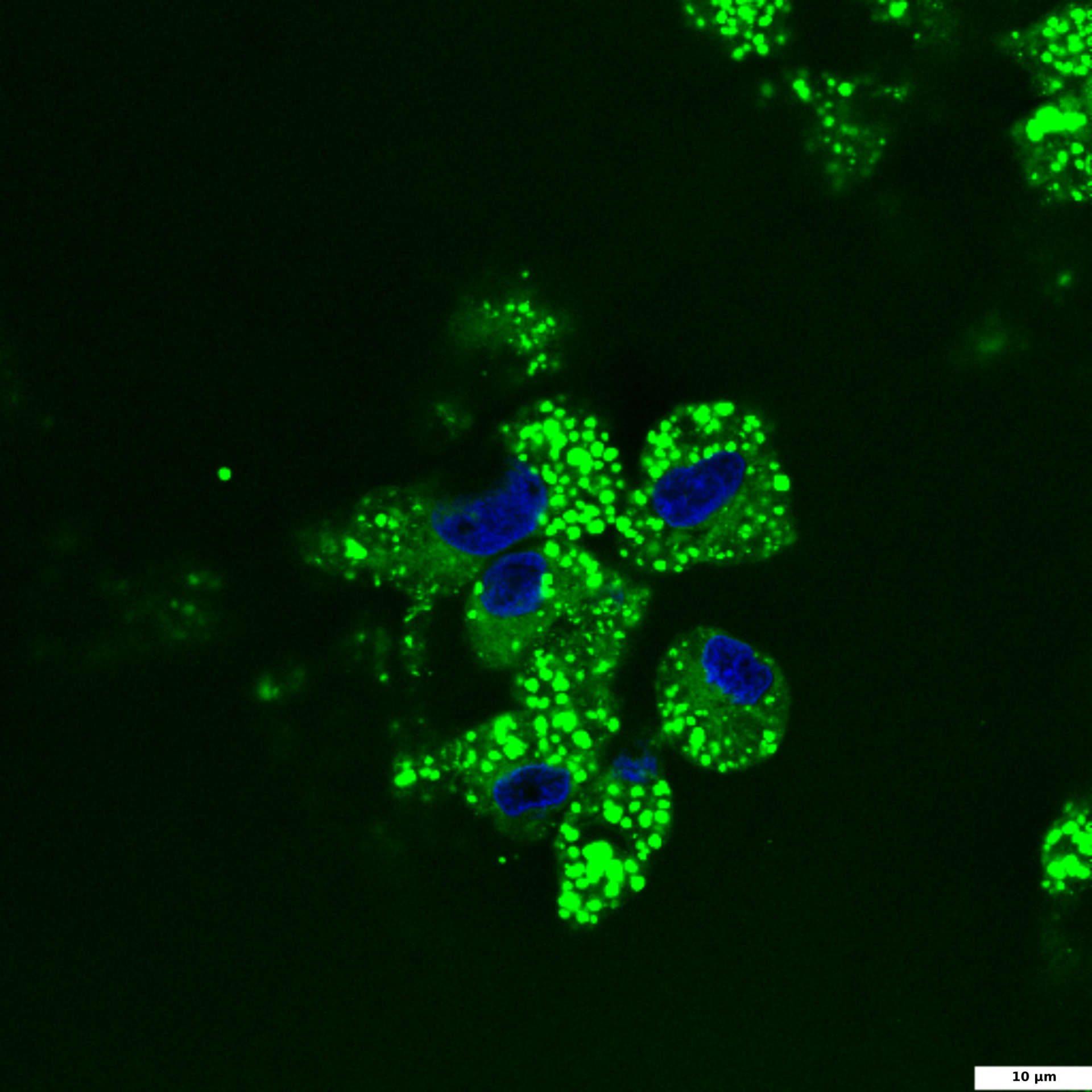
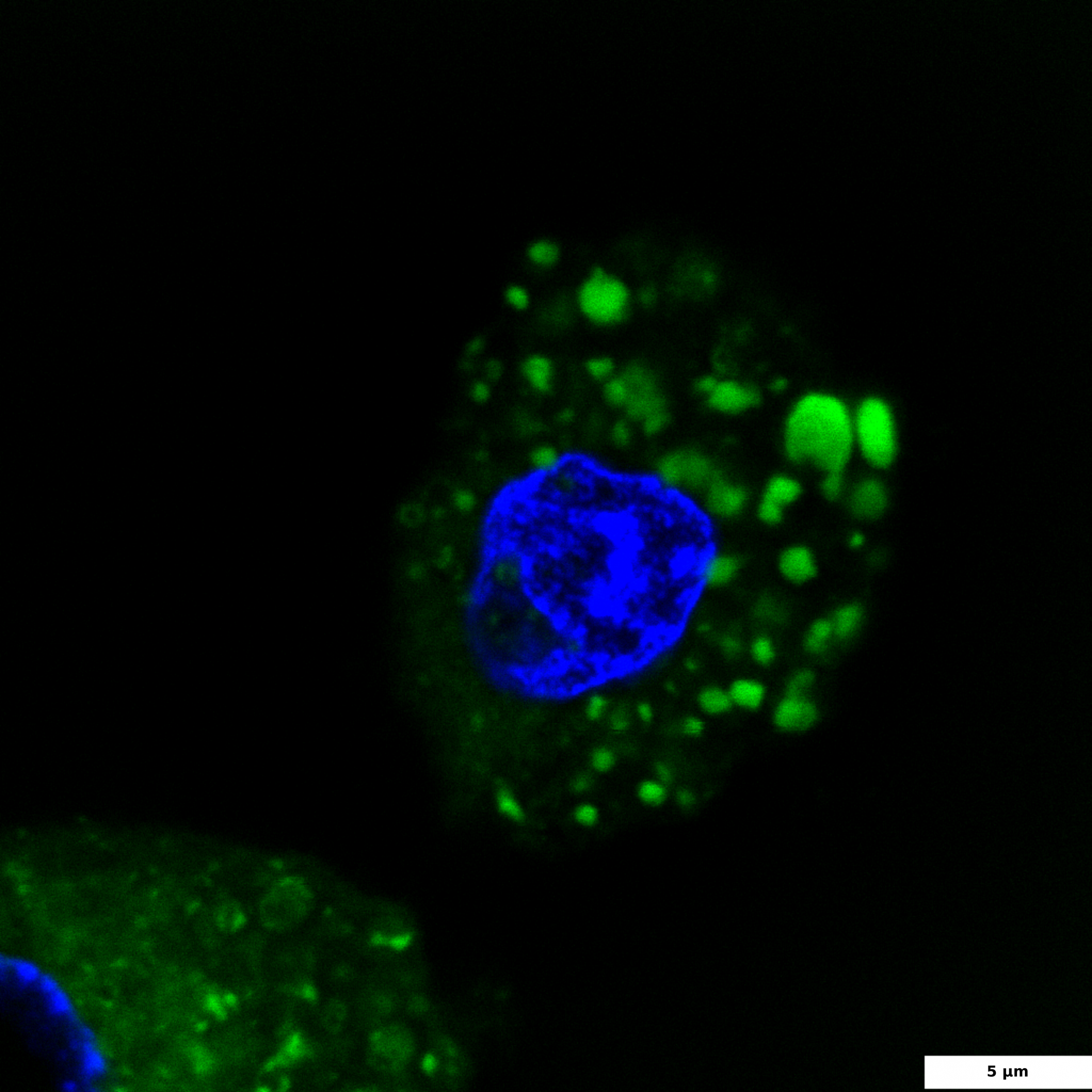
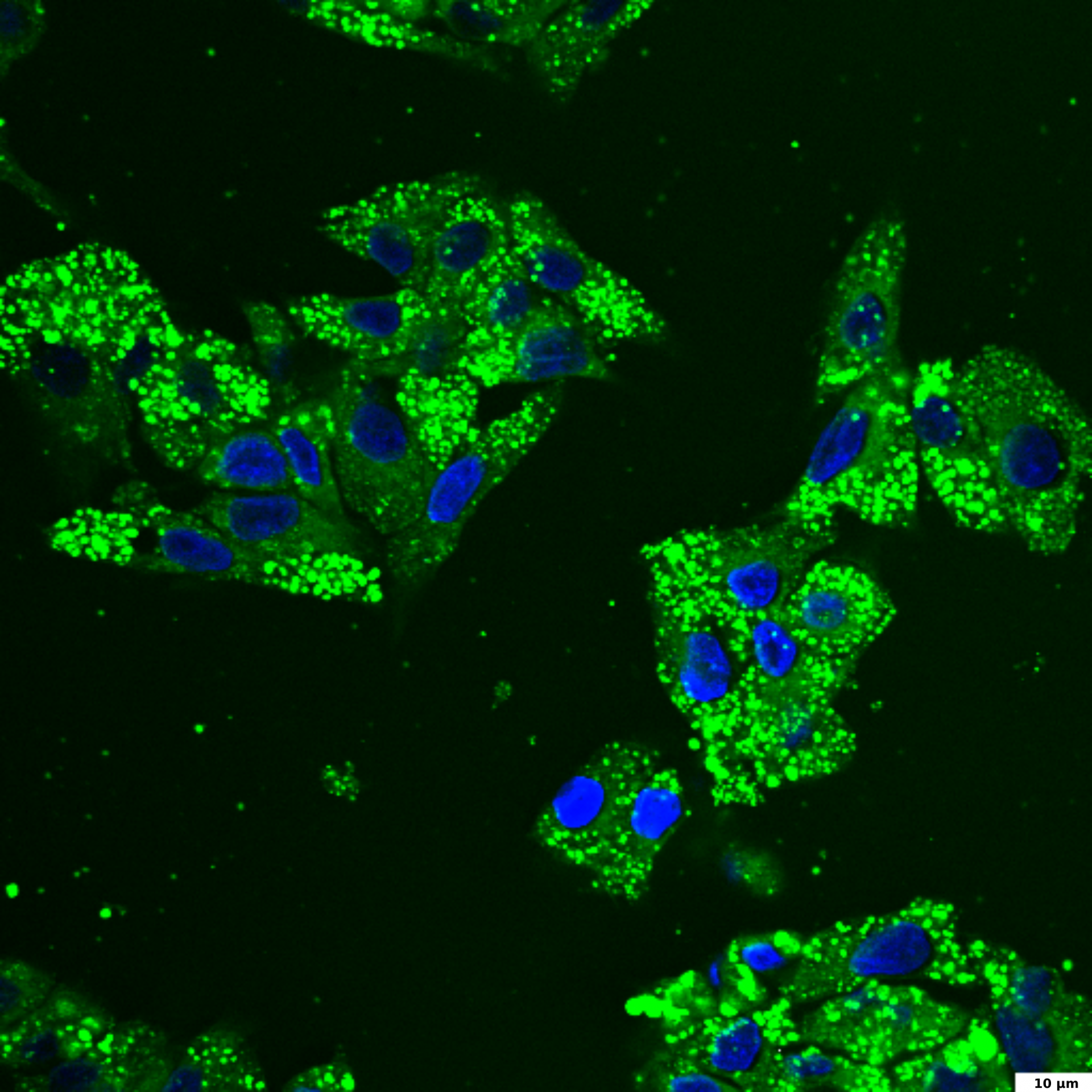
HepG2 culture is a widely used hepatocellular model for the study of liver steatosis. To induce intracellular lipid accumulation, liver cells are treated with free fatty acids (FFA). In our experiment, HepG2 cells were treated with a mixture of oleic and palmitic fatty acids (2:10 ratio) for 24 hours. Steatosis, or increased lipid accumulation, is characterized by the presence of multiple lipid droplets within the cytoplasm of hepatocytes. To visualize lipid droplets by confocal microscopy, cells were fixed and stained with BDP 493/503 (green); DAPI was used for nuclei staining (blue).
BDP 493/503 staining of lipid droplets
HepG2 cells were treated with a 2:10 mixture of oleic and palmitic fatty acids for 24 hours to induce intracellular lipid accumulation. Cells were fixed and stained with BDP 493/503 (green) and DAPI (blue).
The image illustrates DAPI-stained nuclei (blue) and BDP 493/503 stained intracellular lipid droplets (green); 60x and 100x images were captured using a confocal microscope; the scale bar denotes 5 and 10 μm.
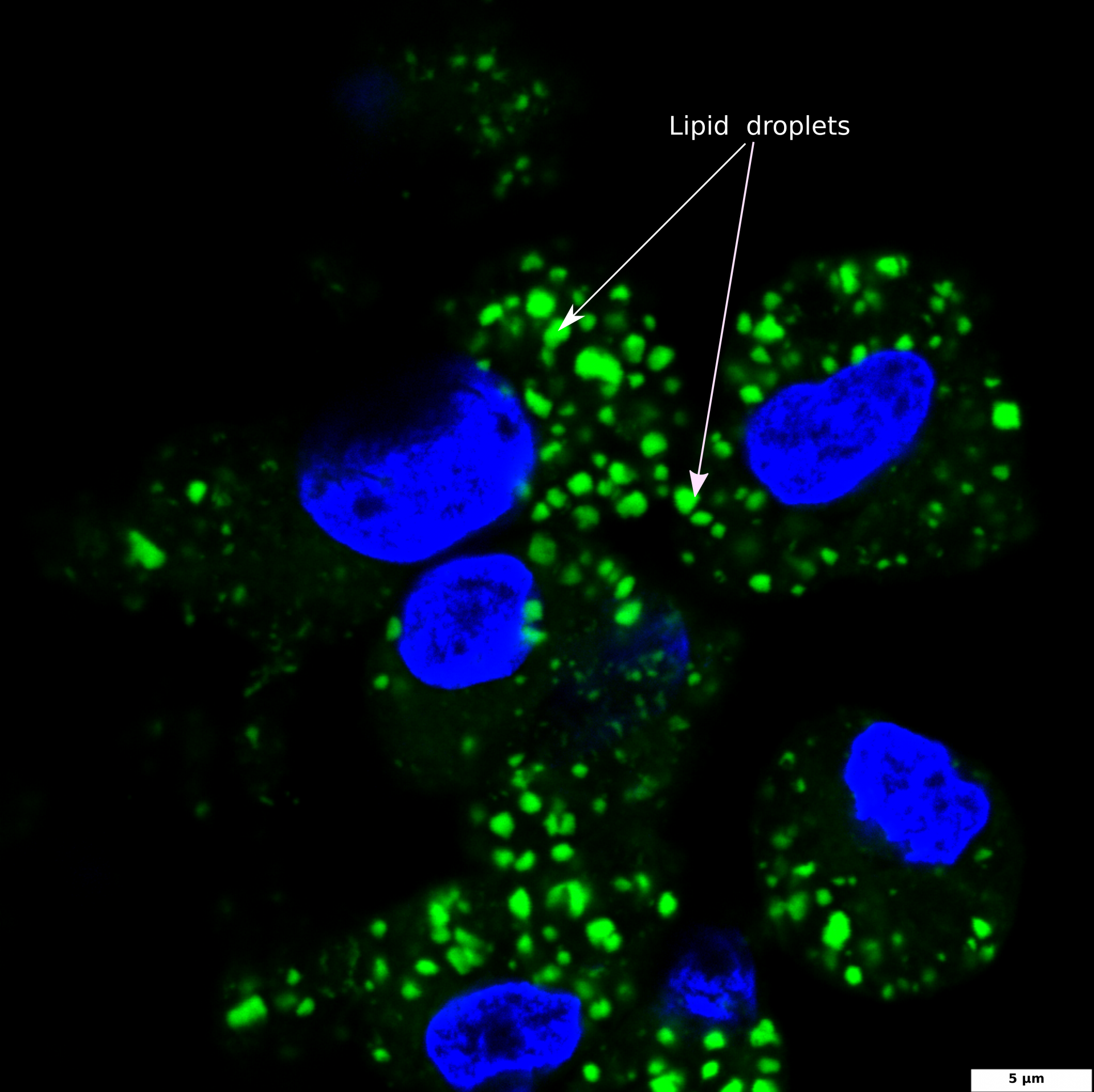
BDP 493/503 staining of lipid droplets
The image illustrates DAPI-stained nuclei (blue) and BDP 493/503 stained intracellular lipid droplets (green); a 100x image was captured using a confocal microscope; the scale bar denotes 5 μm.
Fixing and staining
HepG2 cells were cultured in DMEM supplemented with 10% fetal bovine serum at 37 °C in 5% CO2. Cells were routinely trypsinized (0.05% Trypsin-EDTA) at 70% confluency.
1. Cells were seeded on sterile glass coverslips in a plate/culture dish and cultured overnight so cells were allowed to adhere and equilibrate.
2. Cells were washed twice with pre-warmed PBS and then incubated with DMEM with 10% FBS, 2% BSA, 750 µM FFA for 24 h.
3. The media was removed, and the cells were washed twice with pre-warmed PBS.
4. Cells were fixed in 4% paraformaldehyde (PFA) for 10 minutes at room temperature, followed by three washes with PBS for 5 minutes each.
5. Cells were stained with BDP 493/503 working solution (1-8 µM in PBS) for 20 minutes at 37 °C in the dark, followed by three washes with PBS for 5 minutes each (protected from light).
6. Cells were stained with DAPI working solution for 5 minutes at 37 °C in the dark, followed by three washes with PBS for 5 minutes each (protected from light).
7. Coverslips were removed from the wells and blotted to remove any excess PBS. Coverslips were mounted (cell-side down) on clean microscope slides using the mounting medium.
8. Cells were visualized using a fluorescent microscope/confocal microscope under blue light excitation (488 nm).
Materials
A stock solution of BDP493/503 (5 mM) was prepared by dissolving BDP493/503 (1.3 mg) in DMSO (1 mL). From this stock solution, a sample with a volume of 9.6 µL was taken and diluted in PBS (6 mL) to prepare a working solution with a concentration of 8 µM.
DMEM with 10% FBS, 2% BSA, 750 µM FFA (free fatty acids, oleic acid: palmitic acid = 2:10 ratio)
· A stock solution of FFA (40 mM, oleic acid: palmitic acid = 2:10) was prepared by dissolving oleic acid (1.88 mg, M=282 g/mol) and palmitic acid (8.53 mg, M=256 g/mol) in ethanol (1 mL). The mixture was incubated at +70 °C until the acids had dissolved completely.
· DMEM with 10% FBS, 2% BSA was prepared by adding FA-free BSA (0.8 g) to DMEM with 10% FBS (40 mL). The mixture was incubated at +37 °C until BSA had dissolved completely.
· Final solution (DMEM with 10% FBS, 2% BSA, 750 µM FFA) was obtained by adding 40 mM FFA at +70 °C (750 µL) to DMEM with 10% FBS, 2% BSA at +37 °C (40 mL).
· The final medium was shacked for 30 minutes at +37 °C and filtered before treating the cells.