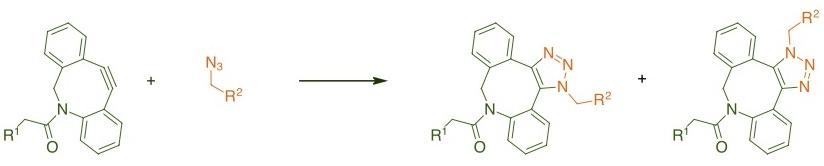
Copper-free click chemistry conjugation using cycloalkyne (DBCO) functionalized dyes is a very robust reaction due to the fact that DBCO and azide derivatives don’t exist in any biological systems. So, this type of conjugation produces minimal background for analytical applications. Due to its simplicity, it can be done by personnel with only general laboratory skills. This reaction gives a stable triazole ring and can be easily used for the conjugation of different biomolecules. Click chemistry conjugates are very stable for prolonged storage at 4°C compared to other chemistries.
Pre-conjugation considerations:
1. Additives like glycerol, NaCl, KCl, potassium phosphate, chelating agents (EDTA, EGTA), and sugars have no effect on conjugation reaction efficiency, but the presence of sodium azide has a great negative impact on the conjugation performance. Buffers can be exchanged by Spin Desalting Columns or dialysis over PBS buffer to remove all undesired additives.
2. For antibody labeling, remove BSA and gelatin additives using MelonTM Gel (Thermo Scientific) or Using Amicon Ultra 0.5 mL Centrifugal Filter Unit with Ultracel 100K membrane (Millipore).
3. For antibody labeling, concentrate the antibody solution after dialysis or purification using Amicon Ultra 0.5mL Centrifugal Filter Unit with Ultracel 10K membrane (Millipore), and recalculate antibody concentration using Nano drop or Bradford protein assay.
4. Prepare fresh 10 mM DBCO-NHS ester solution in DMF or DMSO prior to the labeling. Dissolve crosslinker (4.3 mg) in anhydrous DMSO or DMF (1 mL). DBCO-NHS ester in solid form is stable at -20°C for one year or more but can only be stored for 2-3 months at -20°C when dissolved in DMSO.
5. DMSO and DMF are very hygroscopic liquids that should be protected from exposure to moisture.
Click chemistry antibody-DNA conjugation reaction procedure
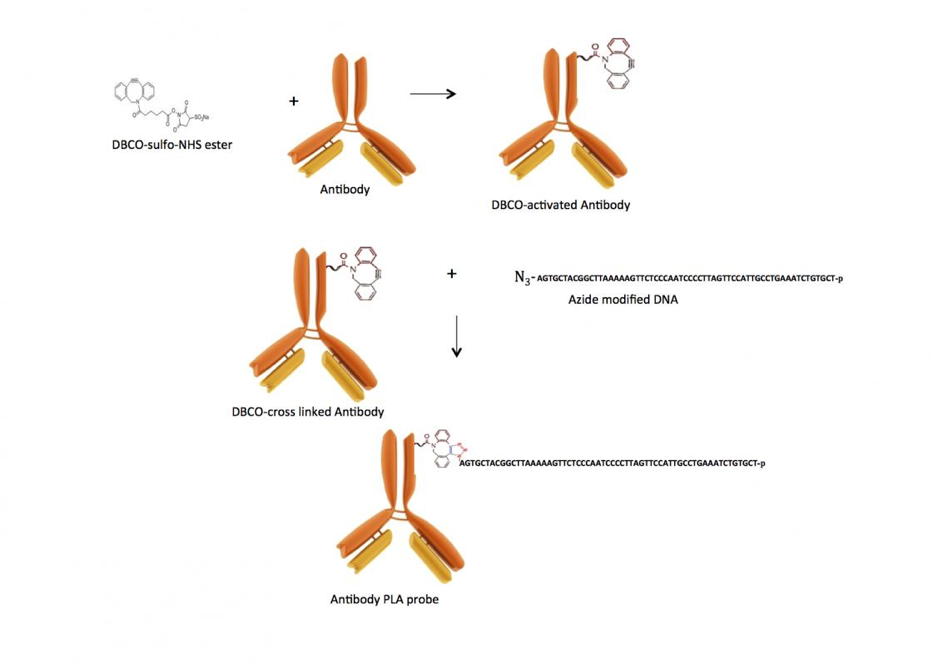
Activation of antibody or another biomolecule with DBCO-NHS ester:
1. Mix antibody with 20-30 fold molar excess of DBCO SE (succinimidyl ester) dissolved in DMSO (a 10 mM solution). DMSO content in the final mixture should be around 20%, and antibody concentration in the reaction mixture should be around 1 mg/mL.
2. Incubate at room temperature for 60 minutes.
Stopping or quenching activation reaction:
1. Add Tris (10 uL, 100mM in water) to the reaction to quench the unreacted DBCO-NHS ester.
2. Incubate for 15 minutes.
3. Remove the unreacted DBCO-NHS ester using a spin desalting column.
Note: DBCO-functionalized antibody can be stored at -20°C for up to a month, but DBCO functional group loses its reactivity over time due to its oxidation and the addition of water to the triple bond.
Copper-Free click reaction.
1. Mix DBCO-functionalized antibody or another biomolecule with 2-4x molar excess of the azide-modified oligonucleotide or azide-functionalized dye.
2. Incubated overnight at 4°C.
3. Validate your final conjugate using SDS gel electrophoresis
4. Remove the unreacted oligonucleotide or dye using liquid chromatography (reverse phase HPLC, ion exchange HPLC, or both).
Selected References:
— Simon et al. (2012) Facile Double-Functionalization of Designed Ankyrin Repeat Proteins using Click and Thiol Chemistries. Bioconjugate Chem. 23(2):279.
— Zeng et al. (2012). 64Cu Core-Labeled Nanoparticles with High Specific Activity via Metal-Free Click Chemistry. ACS Nano. 6(6):5209.
— Arumugam et al. (2011). [18F]Azadibenzocyclooctyne ([18F]ADIBO): A biocompatible radioactive labeling synthon for peptides using catalyst-free [3+2] cycloaddition. Bioorg. Med. Chem. Lett. 21:6987.
— Campbell-Verduyn et al. (2011). Strain-Promoted Copper-Free Click Chemistry for 18F Radiolabeling of Bombesin. Angew. Chem. Int. Ed. 50:11117.
— Debets et al. (2010) Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition. Chem. Commun. 46:97.
— Al-Amin, R. A. Click chemistry antibody-DNA conjugation protocol. The Molecular Methods Database. 2016/01/19
