Strain-promoted azide-alkyne cycloaddition (spAAC) is one of the mild, fast, efficient, and metal-free biorthogonal reactions. In order to leverage this reaction, one must first attach a cycloalkyne group, such as dibenzocyclooctyne (DBCO aka ADIBO), to the molecule of interest. Then, it is possible to conjugate it with an azide. This protocol exemplifies a preparation of a DBCO-labeled antibody.
In this paper, we also determined degrees of labeling of the antibody with different molar excesses of DBCO NHS.
Methods and discussion
10% DMSO in 0.1M sodium bicarbonate was prepared by dissolving sodium bicarbonate (84 mg) in water (9 mL), and adding DMSO (1 mL).
Mouse monoclonal IgG antibody was applied onto Spin-X UF 30K MWCO (Corning) column, and washed three times with 10% DMSO in 0.1M sodium bicarbonate (650 μL) using a centrifuge accoriding to the column manual. Antibody concentration was measured by absorption at 280 nm, and then it was diluted to 1 mg/mL with 10% DMSO in 0.1M sodium bicarbonate. For each reaction, 200 μL of this solution was taken, containing 200 μg (1.33 nmol) of the antibody.
100 mM stock solution of DBCO NHS in DMSO was prepared by dissolving DBCO NHS ester (4.3 mg) in DMSO (100 μL). For each reaction, an aliquot of the stock solution (1 μL) was diluted with DMSO (99 μL) to make 1 mM working solution of DBCO NHS.
8 uL (6 fold molar excess) of 1 mM DBCO NHS working solution in DMSO were added to the antibody solution, mixed well, and the reaction mixture was incubated at room temperature for 1 h. After that, the pure antibody was isolated from the reaction mixtrure using CentriPure P2 Columns (EMP), or similar gel filtration columns, producing a solution of antibody in PBS. In our hands, we obtained 350 μL of a solution with antibody concentration 0.55 mg/mL (antibody yield 193 μg, 96%).
To determine the degree of labeling (DOL), we introduced the purified DBCO antibody into a reaction with an excess of azido dye. Unbound dye was removed, and dye to antibody ratio was determined by spectrophotometry.
To repare a 10 mM solution of sulfo-Cyanine3 azide, 1 mg of dry sulfo-Cyanine3 azide was dissolved in DMSO (138 μL). Then, 1.28 μL of this solution was added to the 350 μL of purified DBCO antibody solution prepared as described above. The mixture was incubated at room temperature overnight. Then, the antibody was isolated from the reaction mixtrure using CentriPure P2 Columns (EMP), or similar gel filtration columns.
The UV spectrum of the solution was measured using Nanodrop or similar spectrophotometer. Absorption spectrum of the labeled antibody contains dye peak (548 nm for sulfo-Cyanine3), and antibody peak (at around 280 nm). To calculate DOL, absorptions at dye absorption maximum (ADye), and at 280 nm (AAB) need to be measured.
DOL is calculated using the following formula:
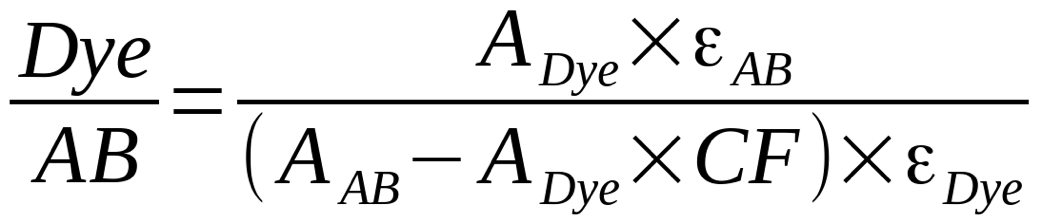
DOL calculation formula for AB labeling reaction.
Dye/AB (or DOL) is the average number of fluorophores per antibody molecule, Adye - optical density of the sample at dye absorption maximum, AAB - sample optical density at antibody absorption maximum (280 nm), εAB is molar extinction coefficient of antibody at 280 nm (210 000 for IgG), εDye is molar extinction coefficient of dye at absorption maximum (162 000 for sulfo-Cyanine3), CF280 is correction factor for particular dye (0.06 for sulfo-Cyanine3).
| DBCO NHS molar excess | Absorbance at 280nm, mAU | Absorbance at 548nm, mAU | Calculated DOL |
| 3 | 162 | 71 | 0.58 |
| 6 | 128 | 136 | 1.47 |
Conclusion
The protocol above demonstrates labeling of the antibody with DBCO NHS ester. The obtained DBCO antibody successfully reacts with dye azide in a copper-free spAAC reaction. It is recommended to use 6-fold molar excess of DBCO NHS to get average degree of labeling around 1.5 molecules of DBCO per antibody.
