In order to answer this question, the following experiment was carried out.
Firstly, we prepared 100 mM stock solutions in DMSO of cyanine dyes: Cyanine3,sulfo-Cyanine3, Cyanine5, and sulfo-Cyanine5 NHS esters.
For comparison purposes, we also prepared 100 mM stock solutions in DMSO of boron-dipyrromethene dyes: BDP TMR and BDP 630/650 NHS esters that have similar fluorescence spectra with Cyanine3 and Cyanine5 NHS esters, respectively.
Secondly, 5 µM solutions of NHS esters were prepared by diluting the stock solutions with mQ water.
Finally, 1 mL of received 5 µM solutions of NHS esters was mixed with 1 mL of buffer solutions in separate tubes (pH: 8.3 - sodium hydrocarbonate, 7.4, 6.2, 4.5, 3.5 - sodium phosphate).
Measurements of the fluorescence emission spectra were taken with a Perkin Elmer LS-55™ fluorometer.
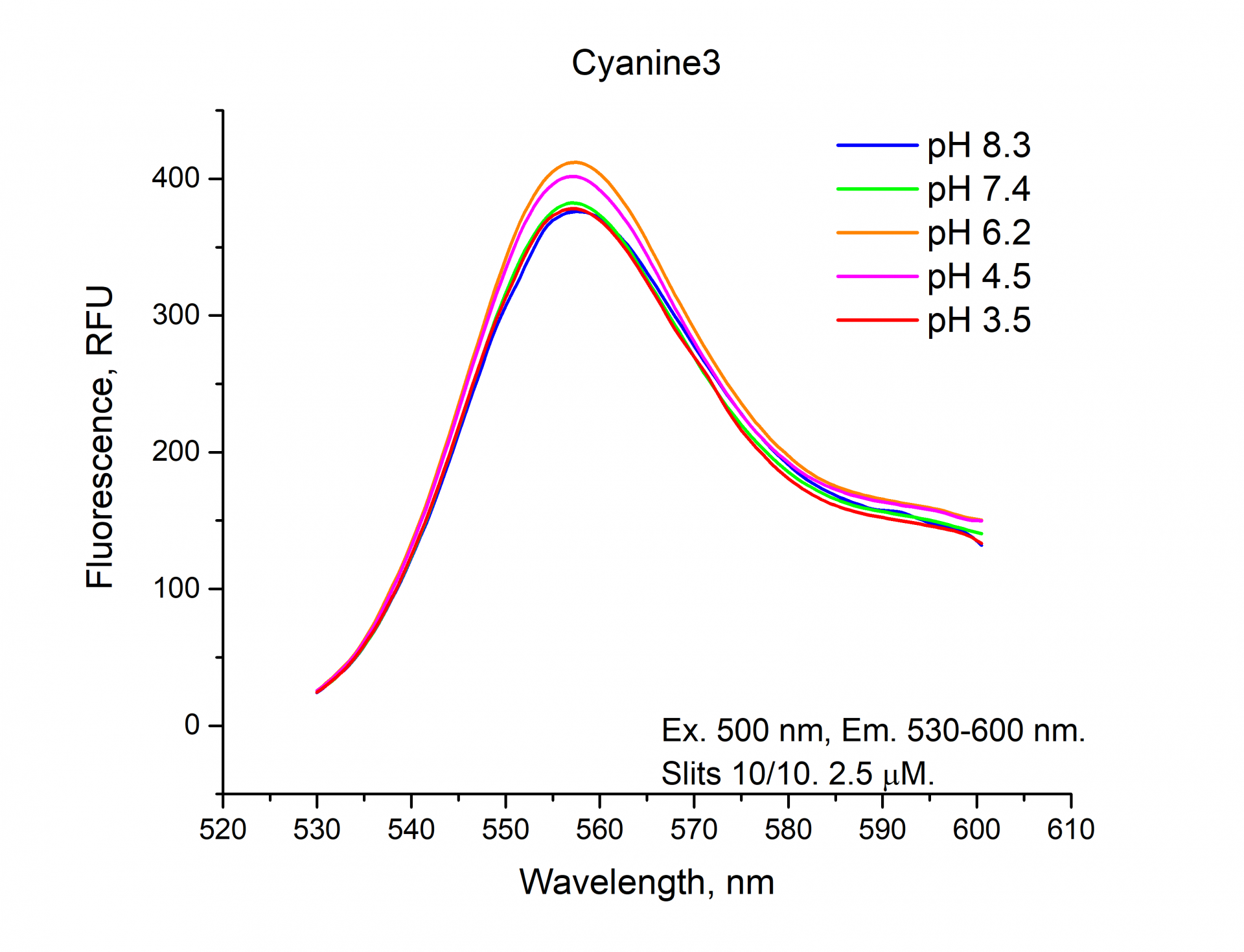
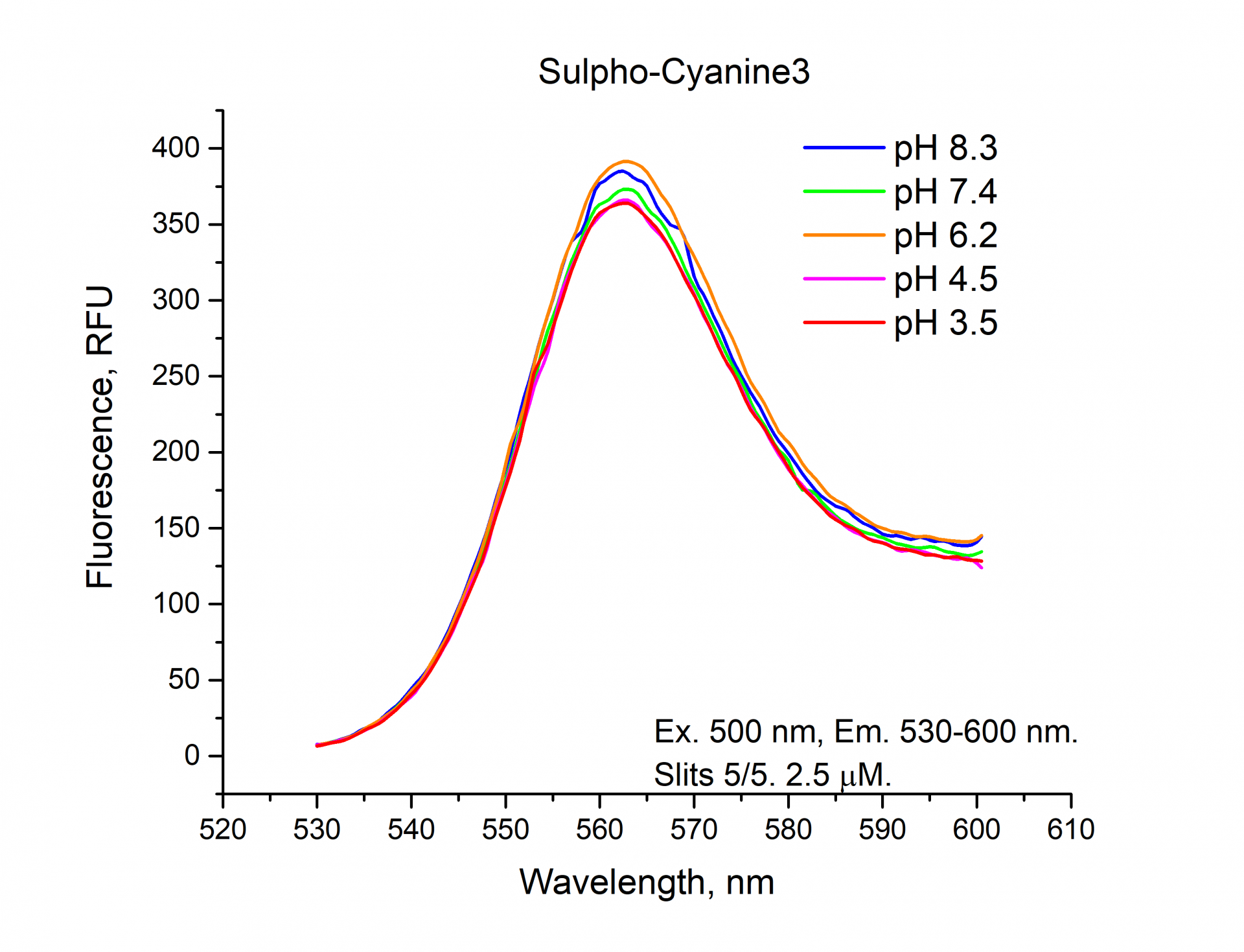
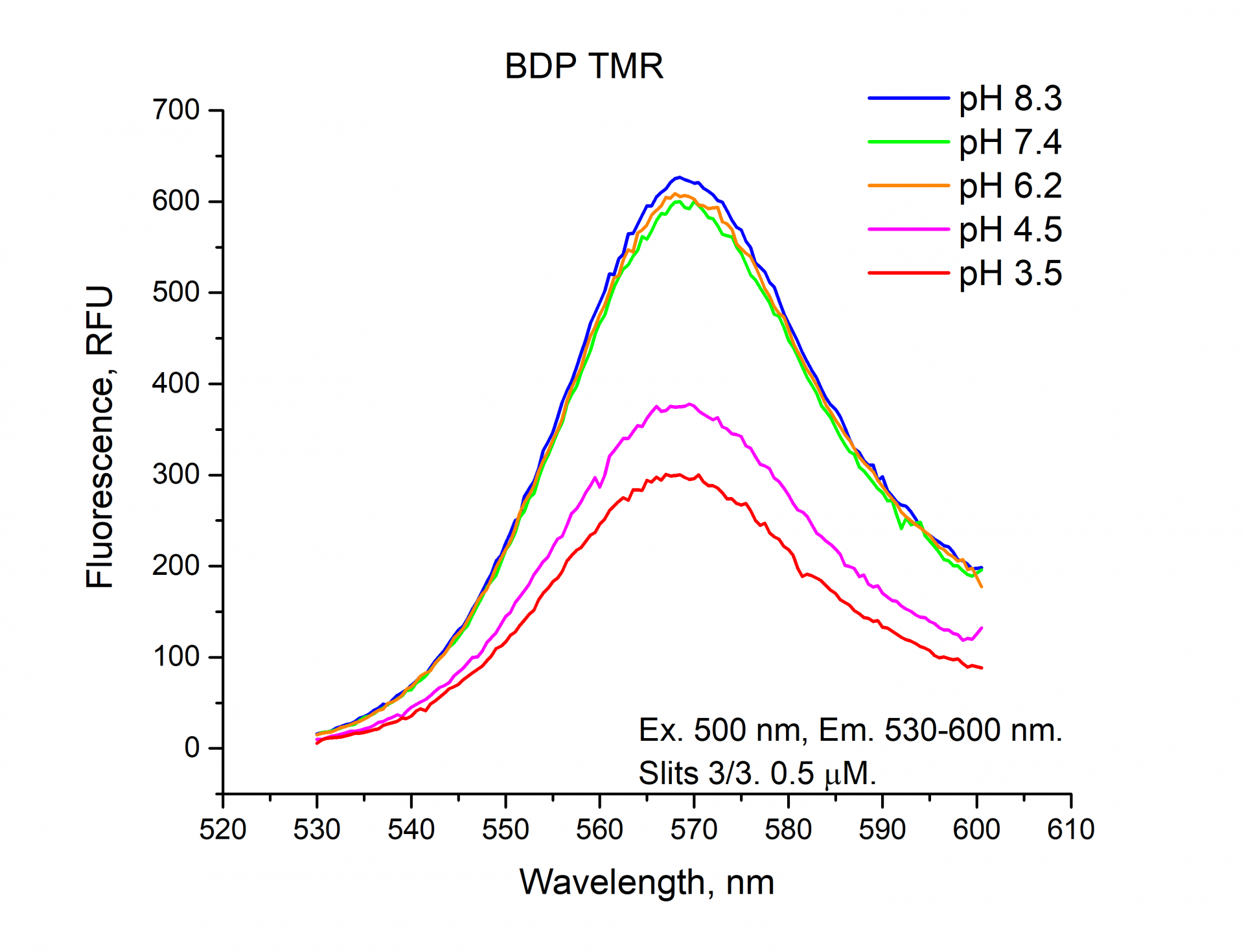
The fluorescence emission spectra of Cyanine3, Sulfo-Cyanine3, BDP TMR NHS esters.
As can be seen from the graphs and a figure, the fluorescence intensity (RFU) of the cyanine dyes Cyanine3 and Sulfo-Cyanine3 is independent of the pH and remains nearly constant (within the range of 5%). Meanwhile, the fluorescence intensity (RFU) of BDP TMR fluorophore does depend on the pH level and decreases in an acidic environment (see pH 3.5, 4.5).
*Due to the very high fluorescence intensity of BDP TMR fluorophore, 5 µM BDP TMR NHS ester solution was diluted 10-fold to a concentration of 0.5 µM to avoid exceeding the instrument measuring range. For the same reason, we don’t see changes in the brightness of fluorescence in tubes with BDP TMR solution in the figure.
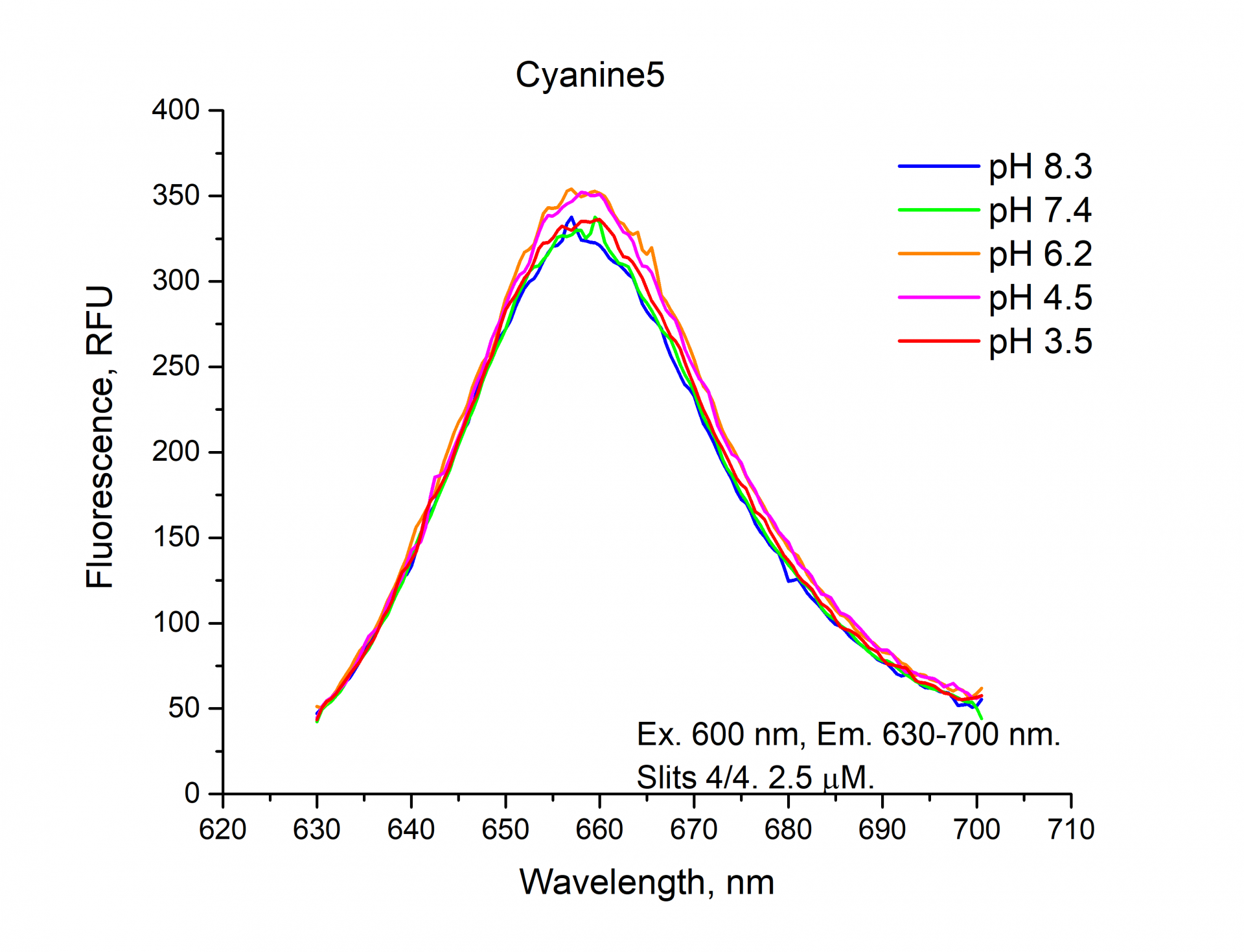
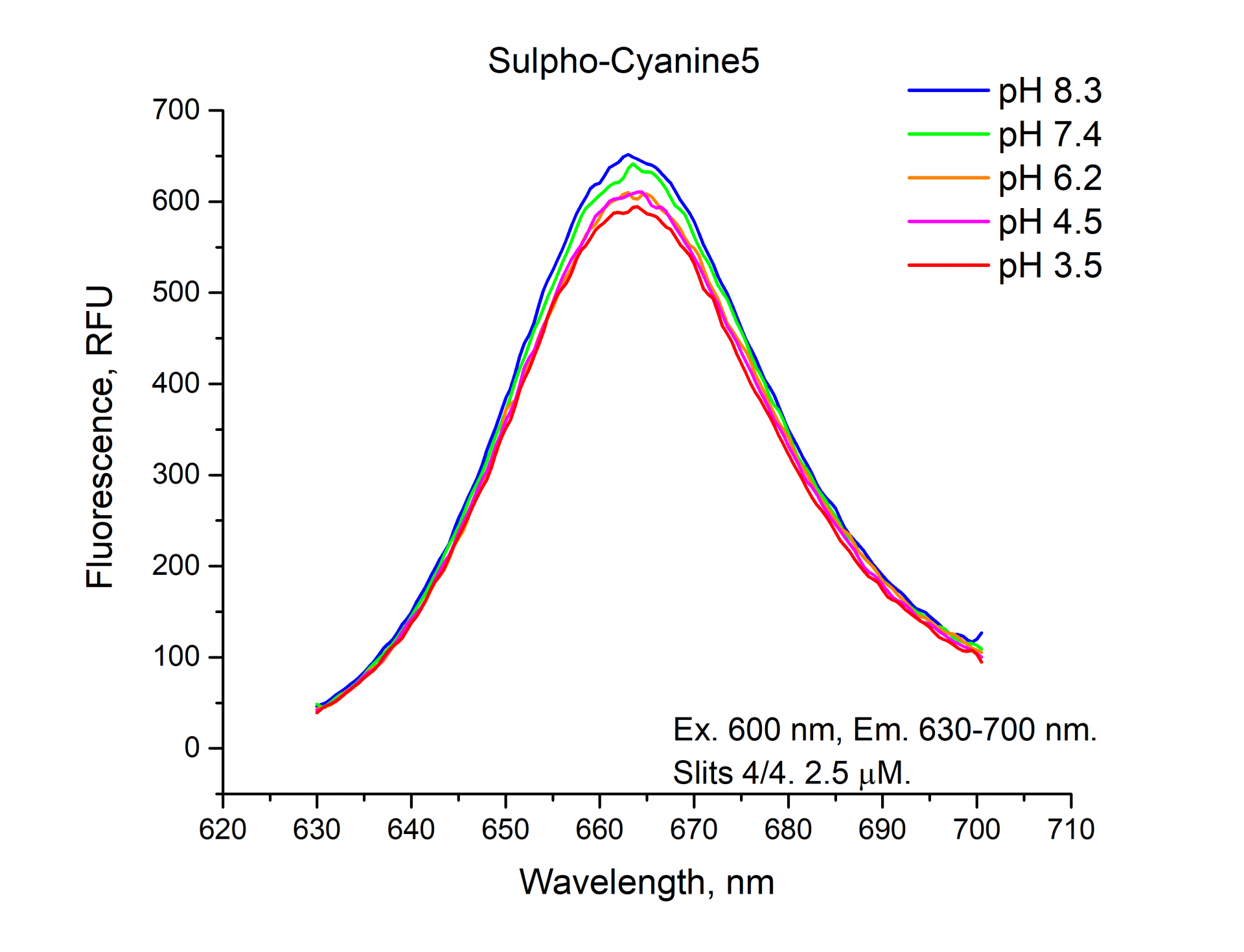
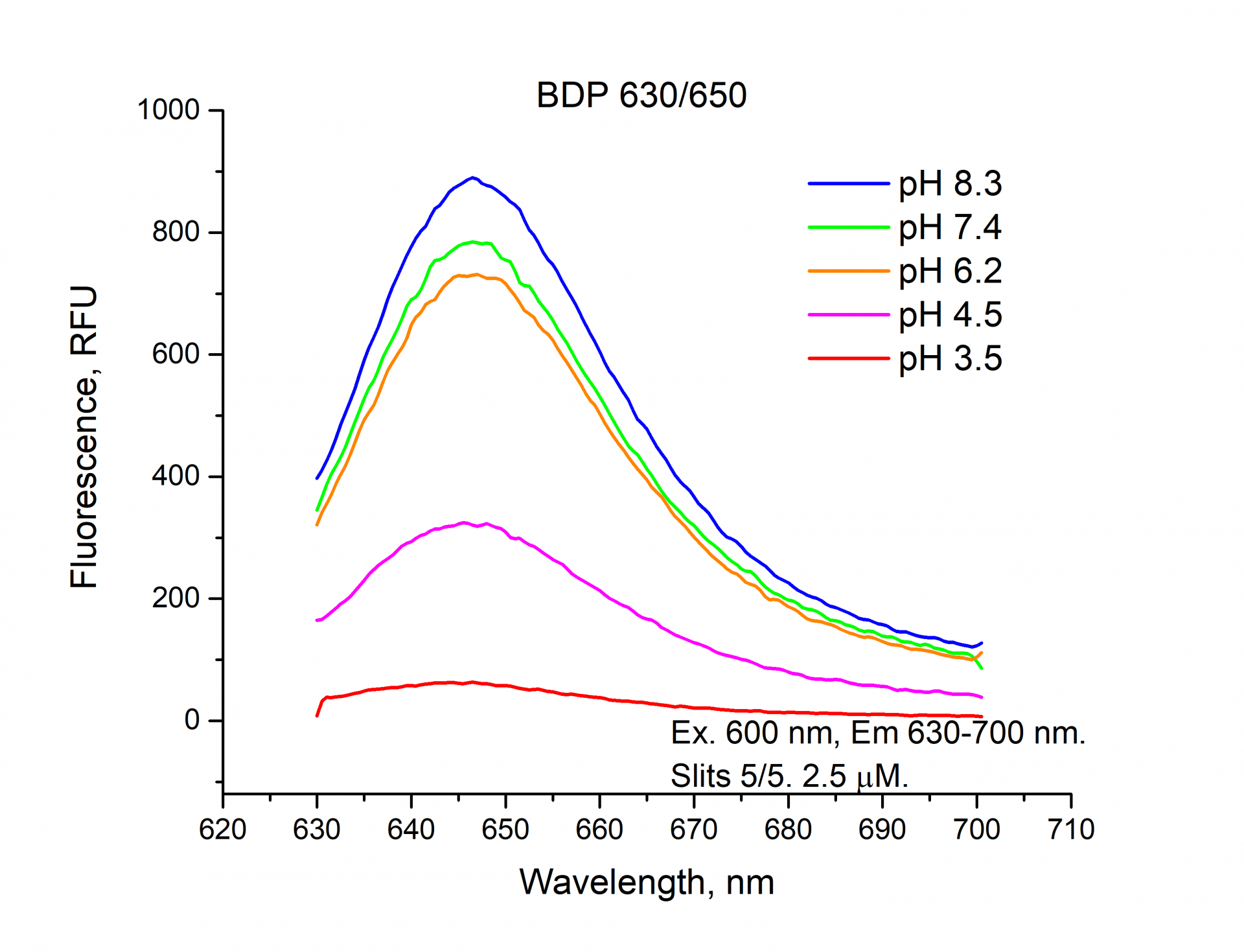
The fluorescence emission spectra of Cyanine5, Sulfo-Cyanine5, BDP 630/650 NHS esters.
Similarly, you can see from the graphs and a figure that the fluorescence intensity (RFU) of the cyanine dyes Cyanine5 and Sulfo-Cyanine5 is independent of the pH and remains nearly constant (within the range of 5%). Meanwhile, the fluorescence intensity (RFU) of BDP 630/650 fluorophore does depend on the pH level and decreases in an acidic environment (see pH 3.5, 4.5).
Summarizing all the above-mentioned information, we can conclude that the fluorescent intensity of the cyanine dyes: Cyanine3, sulfo-Cyanine3, Cyanine5, and sulfo-Cyanine5 NHS esters do not depend on the pH value and remain constant, but the fluorescent intensity of the boron-dipyrromethene dyes: BDP TMR, BDP 630/650 NHS esters depend on pH conditions and decreases significantly in an acidic environment.
Perkin Elmer LS-55™ is trade mark of PerkinElmer.