Hydrazide derivatives can be used to modify glycans and glycoproteins (including antibodies). For this purpose glycan moieties on biomolecules should first be oxidized to form aldehydes. Hydrazides react with aldehydes forming hydrazone bonds.
The oxidation of glycans to aldehydes with sodium periodate depends on pH: the lower the pH is, the more efficient the reaction proceeds [Wolfe & Hage, 1995]. For the oxidation of glycans in hyaluronic acid and antibody, we recommend pH 4.5. In this case, the reaction finishes in 1-2 hours at room temperature and an antibody retains its functionality. After the reaction, it is necessary to destroy a periodate excess using glycerol or propylene glycol.
The reaction of hydrazides with aldehydes to form hydrazones most effectively proceeds at pH 6.0 and takes 2-3 hours at room temperature to complete [Hermanson, 2013] (although a pH range of 5.0-7.4 is acceptable).
Procedure for labeling of hyaluronic acid
1. Prepare a phosphate buffer (100 mM, pH 4.5) by dissolving sodium dihydrogen phosphate monohydrate (0.7 g) in deionized water (50 mL) (it is not necessary to adjust the pH).
2. Dilute hyaluronic acid with the prepared phosphate buffer to a concentration of 0.1%.
3. Prepare a solution of sodium periodate (100 mM) by dissolving sodium periodate (0.021 g) in deionized water (1 mL) (protect from light).
4. Add the 100 mM solution of sodium periodate (10 µL) to 0.1% solution of hyaluronic acid in phosphate buffer pH 4.5* (90 μL) (final concentration is 10 mM), incubate for 2 hours at room temperature.
5. Add ethylene glycol (10 µL) or sodium bisulfite to a final concentration of 10 mM to quench the reaction and destroy an excess of periodate, mix and incubate for 5 minutes at room temperature.
6. Prepare a phosphate buffer (pH 6.0) by dissolving sodium phosphate dibasic heptahydrate (0.37 g) and sodium phosphate monobasic monohydrate (1.19 g) in deionized water (90 mL). Adjust pH of the solution to 6.0 and the final volume of solution to 100 mL.
7. Using gel filtration chromatography remove low molecular weight impurities and exchange phosphate buffer pH 4.5 for phosphate buffer pH 6.0.
8. Dissolve Cyanine5 hydrazide (5 mg) in DMSO (877 µL) to get a 10 mM solution. Add a 10 mM solution of Cyanine5 hydrazide (10-20 µL) to a solution of hyaluronic acid in phosphate buffer pH 6.0*, incubate for 2 hours at room temperature.
9. Using gel filtration chromatography remove an excess of fluorescent dye.
* In case of using other pH values, the reaction time should be increased to 4-6 hours (or overnight).
Procedure for antibody labeling
1. Exchange the buffer, in that an antibody is stored, for a buffer pH 4.5. Dissolve sodium acetate (0.08 g) and sodium chloride (0.435 g) in deionized water (40 mL), adjust the pH to 4.5* using acetic acid, and adjust the volume of solution to 50 mL. For buffer exchange we recommend to use spin concentrators or gel filtration chromatography. Adjust the final antibody concentration to 2 mg/mL.
2. Prepare a solution of sodium periodate (100 mM) by dissolving sodium periodate (0.021 g) in deionized water (1 mL) (protect from light).
3. Add the 100 mM solution of sodium periodate (10 µL) to the antibody solution (90 μL, 180 µg) (final concentration is 10 mM), incubate for 2 hours at room temperature.
4. Add ethylene glycol (10 µL) to quench the reaction and destroy an excess of periodate, mix and incubate for 5 minutes at room temperature.
5. Prepare a phosphate buffer (pH 6.0) by dissolving sodium phosphate dibasic heptahydrate (0.37 g) and Sodium phosphate monobasic monohydrate (1.19 g) in deionized water (90 mL). Adjust pH of the solution to 6.0 and the final volume of solution to 100 mL.
6. Using gel filtration chromatography remove low molecular weight impurities and exchange phosphate buffer pH 4.5 for phosphate buffer pH 6.0*. Measure antibody concentration using absorbance at 280 nm.
7. Dissolve Cyanine5 hydrazide (5 mg) in DMSO (877 µL) to get a 10 mM solution. Add a 10 mM solution of Cyanine5 hydrazide (10x molar excess) to the antibody solution.
For example, if you get 200 µL of antibody solution (0.9 mg/mL, 180 µg=1.2 nmol) after buffer exchange, mix it with the 10 mM solution of Cyanine5 hydrazide (1.2 µL, 10x molar excess = 12 nmol), incubate for 2 hours at room temperature.
8. Using gel filtration chromatography remove the excess of fluorescent dye. Measure the absorbance of antibody solution at 280 and 646 nm, calculate degree of labeling (DOL).
* In case of using other pH values, the reaction time should be increased to 4-6 hours (or overnight).
Using the procedure described above we have got DOL of antibody equal to 1/1.
The UV-visible spectrum of the solution was measured using Nanodrop or similar spectrophotometer. An absorption spectrum of a labeled antibody contains dye peak (646 nm for Cyanine5) and antibody peak (at around 280 nm). DOL is calculated using the following formula:
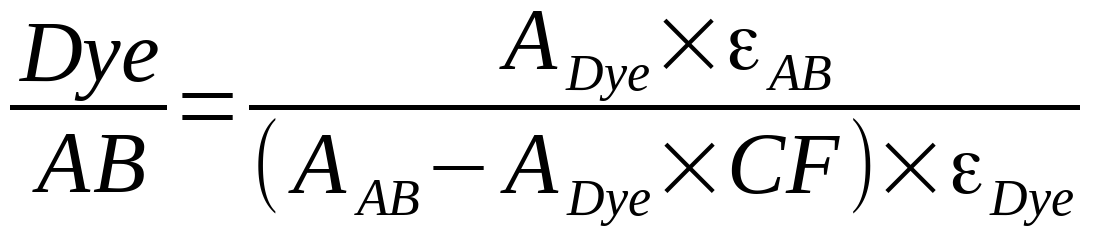
Dye/AB (or DOL) is the average number of fluorophores per antibody molecule, ADye - optical density of the sample at dye absorption maximum, AAB - sample optical density at antibody absorption maximum (280 nm), εAB is molar extinction coefficient of antibody at 280 nm (210 000 for IgG), εDye is molar extinction coefficient of dye at absorption maximum (250 000 for Cyanine5), CF280 is correction factor for particular dye (0.04 for Cyanine5).
Absorbance at 280 nm, AU = 0.812
Absorbance at 646 nm, AU = 0.910
Calculated DOL = 0.98
References
Wolfe CA, Hage DS. Studies on the rate and control of antibody oxidation by periodate. Anal Biochem. 1995 Oct 10;231(1):123-30.
Greg T. Hermanson, Bioconjugate Techniques (Third Edition), Academic Press, 2013, ISBN 9780123822390.

