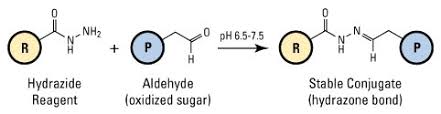
Hydrazides are used for the labeling of molecules bearing carbonyl groups, such as aldehydes and ketones.
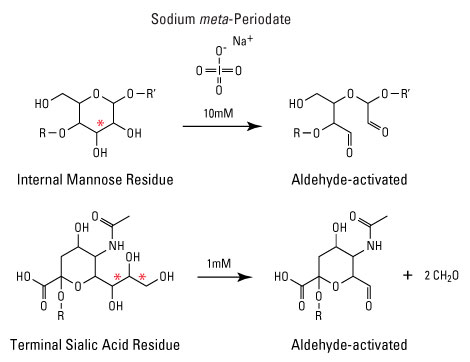
Carbonyl groups can be easily generated from vicinal diol groups of glycoproteins by periodate oxidation - this provides a good method for the labeling of glycoproteins. In the case of antibodies, this is especially useful because the glycosylation site is located far from the epitopes.
We did not place the protocol on our website yet, but it looks as follows:
Omit steps 1-4 if an aldehyde group is already present in the molecule
1. If the molecule does not contain carbonyl groups but contains sugar or another 1,2-diol, cleavage of diol is necessary. Prepare a solution of meta-periodate at 20 mM in 0.1M sodium acetate buffer, pH 5.5. This solution should be used immediately as prepared:
3. Add 1 ml of periodate solution to 1 ml of protein solution. Mix, and incubate for 5 min.
4. Desalt or dialyze against 0.1 M sodium acetate pH 5.5.
5. Prepare hydrazide solution in DMSO (50 mM).
6. Add 200 μl of hydrazide solution to 2 mL of protein solution, and incubate for 2 h at RT.
7. Purify the labeled protein by gel filtration.
The recommended article to read about labeling with hydrazides is Wilchek and Bayer, 1987.
*The images were taken from the ThermoFisher webpage for Carbonyl-reactive Crosslinker Chemistry