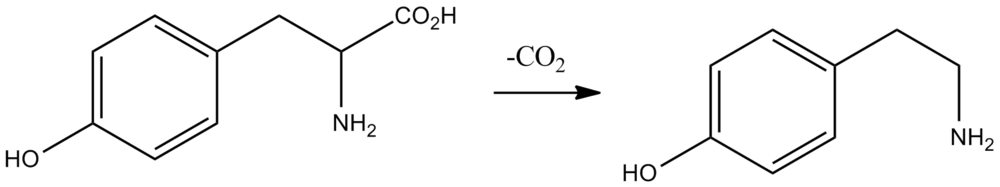
Tyramine is a primary amino compound obtained by formal decarboxylation of the amino acid tyrosine:
Below is the protocol for labeling of tyramine molecule with sulfo-Cyanine3 NHS ester product for use in tyramide signal amplification.
All steps are protected from light and performed at room temperature.
Solution 1: sulfo-Cyanine3 NHS ester 10 mg/mL in Dimethylformamide (DMF) (1 mg in 100 uL).
Solution 2: 1% Triethylamine (TEA) in DMF (10 uL TEA in 1 ml DMF).
Solution 3: 10 mg/mL Tyramine HCl in Solution 2 (10 mg Tyramine HCL in 1 mL Solution2).
Reaction: Mix together 100 uL of Solution 1 + 27 uL of Solution 3; incubate for 4 h at room temp, then add 1.2 mL 100% EtOH.
Results tested side by side with known working fluorescent tyramide conjugates via FISH with digoxigenin-labeled RNA probes and HRP-conjugated anti-digoxigenin antibodies.
The sulfo-Cyanine3-labeled tyramide was tested at several concentrations and showed positive staining similar to control slides.
At the same time, non-sulfonated Cyanine3-labeled tyramide (Cyanine3 NHS ester was used for labeling) was tested at several concentrations and showed no staining whatsoever, while control slides showed normal staining.
The same reaction has been carried out successfully in the past with other Lumiprobe Sulfonated Cyanine NHS ester dyes as well as FAM NHS ester.