BDP® 630/650 amine
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 154C0 | 1 mg |
$125
|
in stock | |
| 254C0 | 5 mg |
$260
|
in stock | |
| 454C0 | 25 mg |
$510
|
in stock | |
| 554C0 | 50 mg |
$895
|
in stock | |
| 654C0 | 100 mg |
$1490
|
in stock |

BDP 630/650 is a far red emitting, borondipyrromethene based fluorophore. The dye is tuned to match the standard Cy5 channel, and can be used as an alternative to Cyanine5 and sulfo-Cyanine5. Compared to cyanines, BDP 630/650 possesses a longer fluorescence lifetime which is important for fluorescence anisotropy measurements.
BDP 630/650 has a brightness similar to cyanines, and an exceptional photostability.
This amine derivative is useful for the reaction with electrophiles, and for enzymatic transamination labeling.
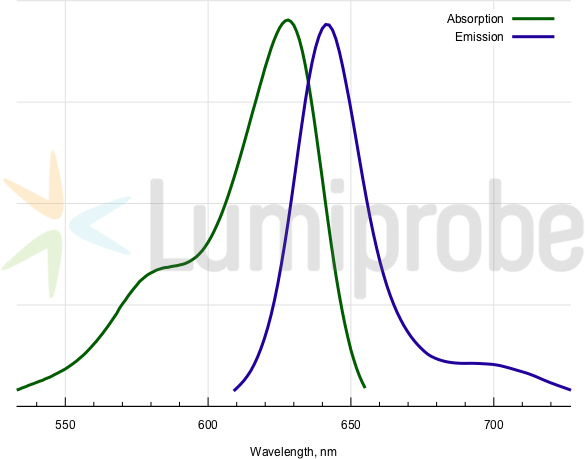
Absorption and emission spectra of BDP 630/650
Customers also purchased with this product
LumiTracker® Mito JC-1
The cationic carbocyanine dye that is widely used as an indicator of mitochondrial membrane potential to study apoptosis and monitor the health of mitochondria.Cyanine3 alkyne
Cyanine3 alkyne is a fluorescent dye alkyne for сlick сhemistry.Cyanine5.5 azide
Far red / near infrared fluorescent Cyanine5.5 dye derivative for click chemistry.General properties
| Appearance: | dark violet solid |
| Molecular weight: | 584.92 |
| Molecular formula: | C29H32N4BClF2O2S |
| Solubility: | moderately soluble in water (81 mM = 47.2 mg/mL), well soluble in DMF, DMSO, alcohols |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 628 |
| ε, L⋅mol−1⋅cm−1: | 97000 |
| Emission maximum, nm: | 642 |
| Fluorescence quantum yield: | 0.91 |
| CF260: | 0.029 |
| CF280: | 0.035 |
Product citations
- Aitova, A.; Scherbina, S.; Berezhnoy, A.; Slotvitsky, M.; Tsvelaya, V.; Sergeeva, T.; Turchaninova, E.; Rybkina, E.; Bakumenko, S.; Sidorov, I.; Popov, M.A.; Dontsov, V.; Agafonov, E.G.; Efimov, A.E.; Agapov, I.; Zybin, D.; Shumakov, D.; Agladze, K. Novel Molecular Vehicle-Based Approach for Cardiac Cell Transplantation Leads to Rapid Electromechanical Graft–Host Coupling. International Journal of Molecular Sciences, 2023, 24(12), 10406. doi: 10.3390/ijms241210406
- Zhang, Y.; Zhu, X.; Chen, X.; Chen, Q.; Zhou, W.; Guo, Q.; Lu, Y.; Li, C.; Zhang, Y.; Liang, D.; Sun, T.; Wei, X.; Jiang, C. Activated Platelets-Targeting Micelles with Controlled Drug Release for Effective Treatment of Primary and Metastatic Triple Negative Breast Cancer. Advanced Functional Materials, 2019, 29(13), 1806620. doi: 10.1002/adfm.201806620
- Zhang, Y.; Guo, Z.; Cao, Z.; Zhou, W.; Zhang, Y.; Chen, Q.; Lu, Y.; Chen, X.; Guo, Q.; Li, C.; Liang, D.; Sun, T.; Jiang, C. Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials, 2018, 183, 243–257. doi: 10.1016/j.biomaterials.2018.06.002
BDP® is a trademark of Lumiprobe
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/3d
The count of items is incorrect.












 $
$ 
