Cyanine3 carboxylic acid
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 11090 | 1 mg |
$110
|
in stock | |
| 21090 | 5 mg |
$210
|
in stock | |
| 41090 | 25 mg |
$410
|
in stock | |
| 51090 | 50 mg |
$695
|
in stock | |
| 61090 | 100 mg |
$1190
|
in stock |

Free Cyanine3 carboxylic acid (Cy3® carboxylic acid analog), non-activated dye. Non-sulfonated reagent, with good solubility in organic solvents and limited aqueous solubility. The dye can be used as a non-reactive fluorophore for control experiments, and for calibration.
For coupling with amines, and labeling, consider using Cyanine3 NHS ester, or water-soluble sulfo-Cyanine3 NHS ester.
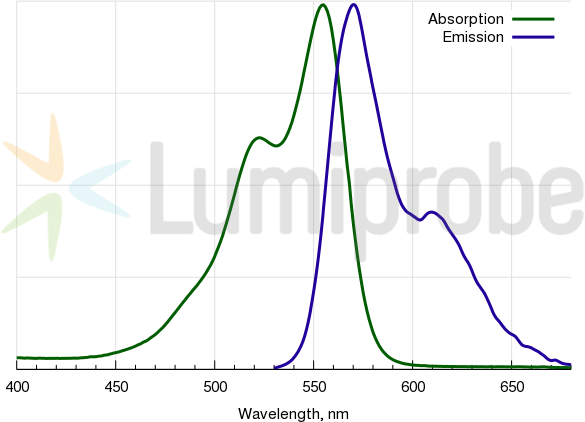
Cyanine3 absorbance and emission spectra
Customers also purchased with this product
Cyanine2 NHS ester minimal dye
Cyanine2 NHS ester minimal dye for protein labeling, an equivalent of Cy2 NHS ester minimal dye.Cyanine3 NHS ester
Amine-reactive Cyanine3 dye NHS ester for the labeling of amino-groups.sulfo-Cyanine3 NHS ester
Water soluble sulfo-Cyanine3 SE activated ester for amino-biomolecule labeling.General properties
| Appearance: | red powder |
| Molecular weight: | 493.08 |
| CAS number: | 1361402-15-4 (inner salt), 1032678-01-5 (chloride), 1251915-29-3 (iodide) |
| Molecular formula: | C30H37ClN2O2 |
| Solubility: | soluble in organic solvents (DMF, DMSO, dichloromethane), poorly soluble in water (1.8 g/L = 4.0 mM) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 555 |
| ε, L⋅mol−1⋅cm−1: | 150000 |
| Emission maximum, nm: | 570 |
| Fluorescence quantum yield: | 0.31 |
| CF260: | 0.04 |
| CF280: | 0.09 |
Product citations
- Herkert, E.K.; Lau, L.; Pons Lanau, R.; Garcia-Parajo, M.F. Hexagonal Plasmonic Arrays for High-Throughput Multicolor Single-Molecule Studies. ACS Applied Materials & Interfaces, 2024, 16(31), 41271-41280. doi: 10.1021/acsami.4c04744
- De Pauw, E.; Chen, Y.; De Keersmaecker, H.; De Coninck, E.; De Smet, L.; De Geest, B.; Braeckmans, K.; Vervaet, C.; Vanhoorne, V. Drying Behaviour and Visualization of Surfactants after Co-Spray Drying of Surfactant-Stabilized Aqueous Suspensions. International Journal of Pharmaceutics, 2023, 643, 123231. doi: 10.1016/j.ijpharm.2023.123231
- Xu, S.; Zhang, P.; Heing-Becker, I.; Zhang, J.; Tang, P.; Bej, R.; Bhatia, S.; Zhong, Y.; Haag, R. Dual Tumor- and Subcellular-Targeted Photodynamic Therapy Using Glucose-Functionalized MoS2 Nanoflakes for Multidrug-Resistant Tumor Ablation. Biomaterials, 2022, 290, 121844. doi: 10.1016/j.biomaterials.2022.121844
- Mattila, J. T.; Beaino, W.; White, A. G.; Nyiranshuti, L.; Maiello, P.; Tomko, J.; Frye, L. J.; Fillmore, D.; Scanga, C. A.; Lin, P. L.; Flynn, J. L.; Anderson, C. J. Retention of 64Cu-FLFLF, a Formyl Peptide Receptor 1-Specific PET Probe, Correlates with Macrophage and Neutrophil Abundance in Lung Granulomas from Cynomolgus Macaques. ACS Infect. Dis., 2021, 7(8), 2264–2276. doi: 10.1021/acsinfecdis.0c00826
Cy® is a trademark of GE Healthcare.
Short link - lumiprobe.com/sh/p/W
The count of items is incorrect.












 $
$ 
