BDP® 630/650-X-NHS ester
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 15420 | 1 mg |
$125
|
5 days | |
| 25420 | 5 mg |
$260
|
in stock | |
| 45420 | 25 mg |
$510
|
in stock | |
| 55420 | 50 mg |
$895
|
in stock | |
| 65420 | 100 mg |
$1490
|
in stock |

BDP 630/650 is a borondipyrromethene fluorophore that has a high molar extinction coefficient, excellent quantum yield, and a relatively long lifetime of the excited state. Due to it, this fluorophore is useful for fluorescence polarization assays that allow to detect binding between molecules.
This is an amine reactive NHS ester. It contains an aminohexanoyl linker between the fluorophore and the reactive group.
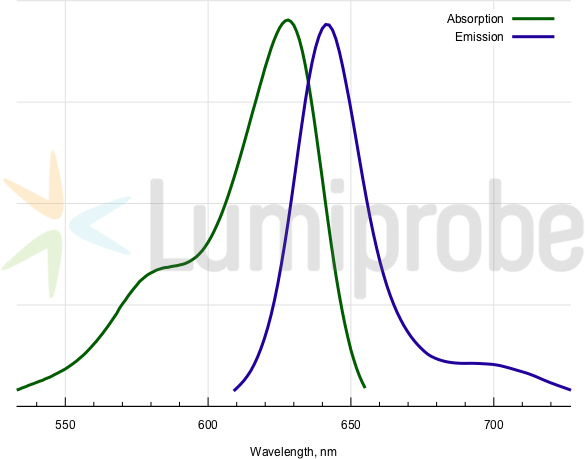
Absorption and emission spectra of BDP 630/650
Customers also purchased with this product
General properties
| Appearance: | dark colored solid |
| Mass spec M+ increment: | 545.2 |
| Molecular weight: | 660.5 |
| CAS number: | 2213445-35-1; 380367-48-6 |
| Molecular formula: | C33H31N4BF2O6S |
| IUPAC name: | Succinimidyl-6-[2-(p-{(E)-2-[4,4-difluoro-5-(2-thienyl)-3a,4a-diaza-4-bora-s-indacen-3-yl]ethenyl}phenoxy)acetylamino]hexanoate |
| Solubility: | good in DMF, DMSO |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 628 |
| ε, L⋅mol−1⋅cm−1: | 97000 |
| Emission maximum, nm: | 642 |
| Fluorescence quantum yield: | 0.91 |
| CF260: | 0.029 |
| CF280: | 0.035 |
Product citations
- Zhao, Z.; Li, C.; Zhang, Y.; Li, C.; Chu, Y.; Li, X.; Liu, P.; Chen, H.; Wang, Y.; Su, B.; Chen, Q.; Sun, T.; Jiang, C. Nanomaterials with Dual Immunomodulatory Functions for Synergistic Therapy of Breast Cancer Brain Metastases. Bioactive Materials, 2023, 27, 474–487. doi: 10.1016/j.bioactmat.2023.04.021
- Casella, B.M.; Farmer, J.P.; Nesheva, D.N.; Williams, H.E.L.; Charlton, S.J.; Holliday, N.D.; Laughton, C.A.; Mistry, S.N. Design, Synthesis, and Application of Fluorescent Ligands Targeting the Intracellular Allosteric Binding Site of the CXC Chemokine Receptor 2. Journal of Medicinal Chemistry, 2023, 66(18), 12911-12930. doi: 10.1021/acs.jmedchem.3c00849
- Brodszkij, E.; Westensee, I. N.; Holleufer, S. F.; Ade, C.; Andres, P. D. D.; Pedersen, J. S.; Städler, B. Membrane Composition of Polymer-Lipid Hybrid Vesicles. Applied Materials Today, 2022, 29, 101549. doi: 10.1016/j.apmt.2022.101549
- Grätz, L.; Tropmann, K.; Bresinsky, M.; Müller, C.; Bernhardt, G.; Pockes, S. NanoBRET binding assay for histamine H2 receptor ligands using live recombinant HEK293T cells. Scientific Reports, 2020, 10, 13288. doi: 10.1038/s41598-020-70332-3
BDP® is a trademark of Lumiprobe
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/3k
The count of items is incorrect.
























 $
$ 
