AF 568 DBCO
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 1158F0 | 1 mg |
$140
|
5 days | |
| 2158F0 | 5 mg |
$450
|
in stock | |
| 4158F0 | 25 mg |
$1460
|
in stock | |
| 5158F0 | 50 mg |
$1890
|
in stock | |
| 6158F0 | 100 mg |
$2990
|
in stock |

Dibenzocyclooctyne (DBCO, DBCO, ADIBO) is one of the most reactive cycloalkynes for copper-free click reaction (SPAAC, strain-promoted azide-alkyne cycloaddition). The rate of interaction of DBCO with azides is significantly higher than that of other cyclooctynes, as well as Cu-catalyzed click reaction (CuAAC). Unlike other cyclooctynes, DBCO does not interact with tetrazines, which makes it possible to use it in bioorthogonal reactions together with trans-cyclooctenes and tetrazines.
AF 568 is a bright, photostable, and hydrophilic fluorophore that emits in the orange channel. The absorption maximum is 572 nm. The emission maximum is 598 nm.
AF 568 DBCO allows fluorescent labeling of azide-containing biomolecules inside living cells and whole organisms without the negative effect of copper ions on them, and inanimate samples.
Absorption and emission spectra of AF 568

Customers also purchased with this product
General properties
| Appearance: | dark violet solid |
| Molecular weight: | 1197.53 |
| Molecular formula: | C66H80N6O11S2 |
| Quality control: | NMR 1H and HPLC-MS (95+%) |
| Storage conditions: | 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Desiccate. Avoid prolonged exposure to light. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 572 |
| ε, L⋅mol−1⋅cm−1: | 94238 |
| Emission maximum, nm: | 598 |
| Fluorescence quantum yield: | 0.912 |























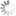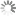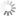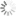
 $
$ 
