H2DCFDA (2′,7′-dichlorodihydrofluorescein diacetate)
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 2247-10mg | 10 mg |
–
|
in stock | |
| 2247-100mg | 100 mg |
$110
|
in stock | |
| 2247-250mg | 250 mg |
$190
|
in stock |

H2DCFDA (2′,7′-dichlorodihydrofluorescein diacetate) is a common reagent used for investigating the production of reactive oxygen species in living cells.
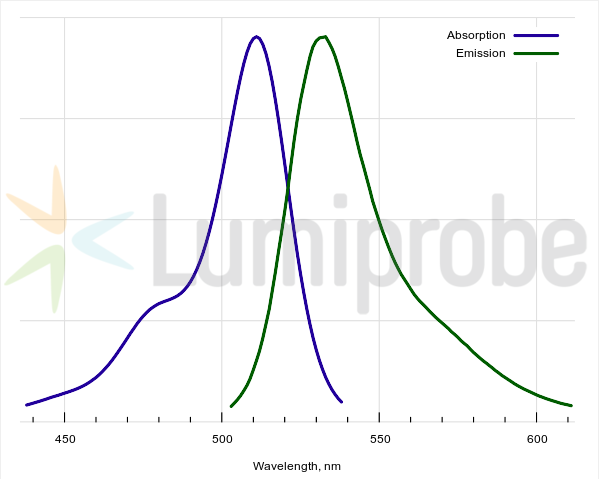
H2DCFDA is a non-fluorescent fluorescein derivative (its reduced acetylated form). The reagent begins to emit fluorescence only after cleavage of acetyl groups and the reagent’s oxidation in the cell while converting to 2’,7’-dichlorofluorescein. This is a bright green-fluorescent dye (absorption maximum 511 nm, fluorescence maximum 533 nm). This reagent can be used for assays in living cells and is not compatible with sample fixation.
Acetyl groups in H2DCFDA increase its lipophilicity and improve its cell membrane permeability. Once got into the cell, the dye is deacetylated by cell esterases, thus becoming charged and better fixed inside the cell. Oxidation with reactive oxygen forms results in the formation of a fluorescent product (2’,7’-dichlorofluorescein) and can be detected using various methods, for example with a flow cytometer, plate reader, or fluorescent microscope.
Recommendations for using the reagent:
- Use a freshly prepared solution of the reagent (the working solution is not intended for long-term storage because of gradual reagent oxidation).
- Select an optimal working concentration of the reagent and incubation time required for reagent deacetylation and oxidation for the specific cell line and assay conditions. If there are no protocols recommended for the specific cell line, start with a concentration from 1 to 10 µM and incubation for 30 min.
- Do not incubate the dye with the cells in the presence of serum because it contains enzymes that cleave H2DCFDA.
H2DCFDA absorbance and emission spectra
Customers also purchased with this product
General properties
| Appearance: | colorless solid |
| Molecular weight: | 487.29 |
| CAS number: | 4091-99-0 |
| Molecular formula: | C24H16Cl2O7 |
| IUPAC name: | 2-(3,6-diacetyloxy-2,7-dichloro-9H-xanthen-9-yl)benzoic acid |
| Solubility: | good in DMSO, ethanol, and DMF |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | 12 months from delivery at −20°C protected from light. Transportation: Up to 3 weeks at room temperature. Protect from moisture. Avoid storage unprotected from light and unnecessary freeze-thaw cycles of the stock solution. If the reagent is used several times, it is strongly recommended to store it in an atmosphere of dry argon or nitrogen. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 511 |
| ε, L⋅mol−1⋅cm−1: | 118626 |
| Emission maximum, nm: | 533 |
| Fluorescence quantum yield: | 0.76 |
| CF260: | 0.17 |
| CF280: | 0.14 |
Product citations
- Moiseeva, N.; Eroshenko, D.; Laletina, L.; Rybalkina, E.; Susova, O.; Karamysheva, A.; Tolmacheva, I.; Nazarov, M.; Grishko, V. The Molecular Mechanisms of Oleanane Aldehyde-β-Enone Cytotoxicity against Doxorubicin-Resistant Cancer Cells. Biology, 2023, 12(3), 415. doi: 10.3390/biology12030415
- Tolmacheva, I.; Beloglazova, Y.; Nazarov, M.; Gagarskikh, O.; Grishko, V. Synthesis and Anticancer Activity of A-Ring-Modified Derivatives of Dihydrobetulin. International Journal of Molecular Sciences, 2023, 24(12), 9863. doi: 10.3390/ijms24129863
























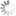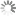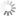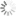
 $
$ 
