Cyanine3 DBCO
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| A10F0 | 1 mg |
$125
|
5 days | |
| B10F0 | 5 mg |
$260
|
in stock | |
| C10F0 | 10 mg |
$325
|
5 days | |
| D10F0 | 25 mg |
$510
|
in stock | |
| E10F0 | 50 mg |
$895
|
in stock | |
| F10F0 | 100 mg |
$1490
|
in stock |

Azodibenzocyclooctyne (DBCO or ADIBO) is a stable but very reactive cycloalkyne for copper-free click chemistry.
This reagent is a conjugate of DBCO with Cyanine3 fluorophore for the non-catalyzed, strain promoted reaction with azides.
Absorption and emission spectra of Cyanine3 fluorophore
.png)
Customers also purchased with this product
General properties
| Appearance: | dark red powder |
| Molecular weight: | 902.99 |
| CAS number: | 2692677-79-3 |
| Molecular formula: | C51H57N4O2PF6 |
| Solubility: | good in DMF, DMSO, DCM, alcohols, practically insoluble in water (< 1 uM, < 1 mg/L) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 555 |
| ε, L⋅mol−1⋅cm−1: | 150000 |
| Emission maximum, nm: | 570 |
| Fluorescence quantum yield: | 0.31 |
Product citations
- Tsuchiya, M.; Tachibana, N.; Hamachi, I. Flow cytometric analysis of phosphatidylcholine metabolism using organelle-selective click labeling. STAR Protocols, 2023, 4(3), 102525. doi: 10.1016/j.xpro.2023.102525
- Jiang, Z.; He, H.; Liu, H.; Thayumanavan, S. Azide-Terminated RAFT Polymers for Biological Applications. Current Protocols in Chemical Biology, 2020, 12(4), e85. doi: 10.1002/cpch.85
- Islam, M.R.; Nguy, C.; Pandit, S.; Lyon, L.A. Design and Synthesis of Core–Shell Microgels with One‐Step Clickable Crosslinked Cores and Ultralow Crosslinked Shells. Macromolecular Chemistry and Physics, 2020, 221(19), 2000156. doi: 10.1002/macp.202000156
- Jiang, Z.; He, H.; Liu, H.; Thayumanavan, S. Cellular Uptake Evaluation of Amphiphilic Polymer Assemblies: Importance of Interplay between Pharmacological and Genetic Approaches. Biomacromolecules, 2019, 20(12), 4407–4418. doi: 10.1021/acs.biomac.9b01073
Short link - lumiprobe.com/sh/p/3C
The count of items is incorrect.
























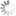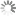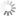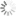
 $
$ 
