Cyanine5 DBCO
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| A30F0 | 1 mg |
$125
|
in stock | |
| B30F0 | 5 mg |
$260
|
in stock | |
| C30F0 | 10 mg |
$325
|
in stock | |
| D30F0 | 25 mg |
$510
|
in stock | |
| E30F0 | 50 mg |
$895
|
in stock | |
| F30F0 | 100 mg |
$1490
|
in stock |

A derivative of Cyanine5 red-emitting fluorophore possessing DBCO (dibenzocyclooctyne, also known as ADIBO, azodibenzocyclooctyne) group for copper free click chemistry.
Strained cycloalkynes, such as cyclooctynes, react with azides very rapidly in the absence of copper catalyst in a strain-promoted alkyne-azide cycloaddition (SPAAC). This reaction is very fast, mild, and biocompatible.
Compared to other cycloalkynes, DBCO provide among the fastest reaction kinetics, still possessing good stability.
Absorption and emission spectra of Cyanine5

Customers also purchased with this product
General properties
| Appearance: | dark blue solid |
| Mass spec M+ increment: | 928.4 |
| Molecular weight: | 929.03 |
| Molecular formula: | C53H59N4F6O2P |
| Solubility: | good in DMF, DMSO, chlorinated organic solvents, practically insoluble in water (<1 uM, < 1 mg/L) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 646 |
| ε, L⋅mol−1⋅cm−1: | 250000 |
| Emission maximum, nm: | 662 |
| Fluorescence quantum yield: | 0.2 |
Product citations
- Toro-González, M.; Akingbesote, N.; Bible, A.; Pal, D.; Sanders, B.; Ivanov, A. S.; Jansone-Popova, S.; Popovs, I.; Benny, P.; Perry, R.; Davern, S. Development of 255Ac-Doped Biocompatible Nanoparticles for Targeted Alpha Therapy. Journal of Nanobiotechnology, 2024, 22(1), 306. doi: 10.1186/s12951-024-02520-6
- Wang, Y.-J.; Li, L.; Yu, J.; Hu, H.-Y.; Liu, Z.-X.; Jiang, W.-J.; Xu, W.; Guo, X.-P.; Wang, F.-S.; Sheng, J.-Z. Imaging of Escherichia Coli K5 and Glycosaminoglycan Precursors via Targeted Metabolic Labeling of Capsular Polysaccharides in Bacteria. Sci. Adv., 2023, 9(7), eade4770. doi: 10.1126/sciadv.ade4770
- Borner, T.; Tinsley, I. C.; Milliken, B. T.; Doebley, S. A.; Najjar, N. R.; Kerwood, D. J.; De Jonghe, B. C.; Hayes, M. R.; Doyle, R. P. Creation of a Peptide Antagonist of the GFRAL–RET Receptor Complex for the Treatment of GDF15-Induced Malaise. J. Med. Chem., 2023, 66(16), 11237–11249. doi: 10.1021/acs.jmedchem.3c00667
- Miranda, A.; Lopez-Blanco, R.; Lopes-Nunes, J.; Melo, A.M.; Campello, M.P.C.; Paulo, A.; Oliveira, M.C.; Mergny, J.-L.; Oliveira, P.A.; Fernandez-Megia, E.; Cruz, C. Gallic Acid–Triethylene Glycol Aptadendrimers Synthesis, Biophysical Characterization and Cellular Evaluation. Pharmaceutics, 2022, 14(11), 2456. doi: 10.3390/pharmaceutics14112456
Cy® is a trademark of GE Healthcare.
Short link - lumiprobe.com/sh/p/36
The count of items is incorrect.
























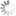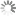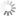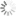
 $
$ 
