endo-BCN CE-phosphoramidite
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
|
3005-100mg
/ in 6 mL septum
|
100 mg |
$390
|
in stock | |
|
3005-250mg
/ in 6 mL septum
|
250 mg |
$850
|
in stock | |
|
3005-1g
/ in 6 mL septum
|
1 g |
$2380
|
in stock |

Bicyclononyne (BCN) is a stable and one of the most reactive cyclooctynes for copper-free click chemistry. Unlike dibenzocyclooctyne (DBCO), BCN is reactive both to azides (strain-promoted azyde-alkyne cycloaddition, SPAAC) and tetrazines (inverse electron demand Diels-Alder reaction, IEDDA). Being an endo-stereoisomer, bicyclononine in endo-BCN CE-phosphoramidite provides not significantly different rate of cycloaddition compared to its exo-conformer.
BCN-labeled oligonucleotides may be used for the conjugation to azide- or tetrazine-containing solid surfaces, polymers, and large proteins.
Coupling time is standard, like for amidites of natural nucleosides. Exclude the dimethoxytrityl (DMT) removal step and use the Dmt-ON protocol after amidite coupling and oxidation.
Use standard conditions for deprotection and ammonia solution, or AMA mixture (ammonium hydroxide / 40% methylamine, 1:1).
Customers also purchased with this product
General properties
| Appearance: | yellowish oil |
| Mass spec M+ increment: | 343.11 |
| Molecular weight: | 481.57 |
| CAS number: | 1352811-59-6 |
| Molecular formula: | C24H40F1N3O5P |
| Solubility: | good in acetonitrile |
| Quality control: | NMR 1H and 31P (95 %) |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Oligo synthesis details
| Diluent: | Anhydrous Acetonitrile |
| Deprotection conditions: | identical to protected nucleobases |























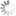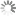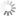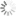
 $
$ 
